Lateral septum adenosine A2A receptors control stress-induced depressive-like behaviors via signaling to the hypothalamus and habenula
- PMID: 37019936
- PMCID: PMC10076302
- DOI: 10.1038/s41467-023-37601-x
Lateral septum adenosine A2A receptors control stress-induced depressive-like behaviors via signaling to the hypothalamus and habenula
Abstract
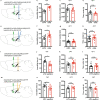
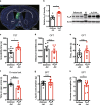
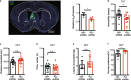
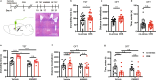
Major depressive disorder ranks as a major burden of disease worldwide, yet the current antidepressant medications are limited by frequent non-responsiveness and significant side effects. The lateral septum (LS) is thought to control of depression, however, the cellular and circuit substrates are largely unknown. Here, we identified a subpopulation of LS GABAergic adenosine A2A receptors (A2AR)-positive neurons mediating depressive symptoms via direct projects to the lateral habenula (LHb) and the dorsomedial hypothalamus (DMH). Activation of A2AR in the LS augmented the spiking frequency of A2AR-positive neurons leading to a decreased activation of surrounding neurons and the bi-directional manipulation of LS-A2AR activity demonstrated that LS-A2ARs are necessary and sufficient to trigger depressive phenotypes. Thus, the optogenetic modulation (stimulation or inhibition) of LS-A2AR-positive neuronal activity or LS-A2AR-positive neurons projection terminals to the LHb or DMH, phenocopied depressive behaviors. Moreover, A2AR are upregulated in the LS in two male mouse models of repeated stress-induced depression. This identification that aberrantly increased A2AR signaling in the LS is a critical upstream regulator of repeated stress-induced depressive-like behaviors provides a neurophysiological and circuit-based justification of the antidepressant potential of A2AR antagonists, prompting their clinical translation.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Comment in
-
Adenosine A2A receptor-bearing GABAergic neurons in the lateral septum of the brain: novel mediators of depressive-like behavior.Purinergic Signal. 2024 Jun;20(3):209-211. doi: 10.1007/s11302-023-09946-x. Epub 2023 May 31. Purinergic Signal. 2024. PMID: 37254004 Free PMC article. No abstract available.
References
-
- Depression and Other Common Mental Disorders: Global Health Estimates. Geneva:World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO.
-
- Mental health atlas 2017. Geneva: World Health Organization; 2018. Licence: CC BY-NC-SA 3.0 IGO.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

