The NK cell receptor NKp46 recognizes ecto-calreticulin on ER-stressed cells
- PMID: 37020026
- PMCID: PMC10165876
- DOI: 10.1038/s41586-023-05912-0
The NK cell receptor NKp46 recognizes ecto-calreticulin on ER-stressed cells
Erratum in
-
Publisher Correction: The NK cell receptor NKp46 recognizes ecto-calreticulin on ER-stressed cells.Nature. 2023 Jun;618(7964):E17. doi: 10.1038/s41586-023-06151-z. Nature. 2023. PMID: 37198495 No abstract available.
Abstract
Natural killer (NK) cell kill infected, transformed and stressed cells when an activating NK cell receptor is triggered1. Most NK cells and some innate lymphoid cells express the activating receptor NKp46, encoded by NCR1, the most evolutionarily ancient NK cell receptor2,3. Blockage of NKp46 inhibits NK killing of many cancer targets4. Although a few infectious NKp46 ligands have been identified, the endogenous NKp46 cell surface ligand is unknown. Here we show that NKp46 recognizes externalized calreticulin (ecto-CRT), which translocates from the endoplasmic reticulum (ER) to the cell membrane during ER stress. ER stress and ecto-CRT are hallmarks of chemotherapy-induced immunogenic cell death5,6, flavivirus infection and senescence. NKp46 recognition of the P domain of ecto-CRT triggers NK cell signalling and NKp46 caps with ecto-CRT in NK immune synapses. NKp46-mediated killing is inhibited by knockout or knockdown of CALR, the gene encoding CRT, or CRT antibodies, and is enhanced by ectopic expression of glycosylphosphatidylinositol-anchored CRT. NCR1)-deficient human (and Nrc1-deficient mouse) NK cells are impaired in the killing of ZIKV-infected, ER-stressed and senescent cells and ecto-CRT-expressing cancer cells. Importantly, NKp46 recognition of ecto-CRT controls mouse B16 melanoma and RAS-driven lung cancers and enhances tumour-infiltrating NK cell degranulation and cytokine secretion. Thus, NKp46 recognition of ecto-CRT as a danger-associated molecular pattern eliminates ER-stressed cells.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Figures



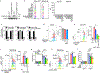




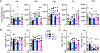
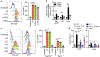


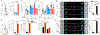

Comment in
-
Calreticulin surface presentation: a signal for natural killer cells to attack.Signal Transduct Target Ther. 2023 Aug 7;8(1):289. doi: 10.1038/s41392-023-01551-z. Signal Transduct Target Ther. 2023. PMID: 37544934 Free PMC article. No abstract available.
References
-
- Sivori S et al. NKp46 is the major triggering receptor involved in the natural cytotoxicity of fresh or cultured human NK cells. Correlation between surface density of NKp46 and natural cytotoxicity against autologous, allogeneic or xenogeneic target cells. Eur. J. Immunol 29, 1656–1666 (1999). - PubMed
-
- Obeid M et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med 13, 54–61 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

