Strategies to minimise and monitor biases and imbalances by arm in surgical cluster randomised trials: evidence from ChEETAh, a trial in seven low- and middle-income countries
- PMID: 37020311
- PMCID: PMC10077601
- DOI: 10.1186/s13063-022-06852-2
Strategies to minimise and monitor biases and imbalances by arm in surgical cluster randomised trials: evidence from ChEETAh, a trial in seven low- and middle-income countries
Abstract
Background: Cluster randomised controlled trials (cRCT) present challenges regarding risks of bias and chance imbalances by arm. This paper reports strategies to minimise and monitor biases and imbalances in the ChEETAh cRCT.
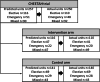
Methods: ChEETAh was an international cRCT (hospitals as clusters) evaluating whether changing sterile gloves and instruments prior to abdominal wound closure reduces surgical site infection at 30 days postoperative. ChEETAh planned to recruit 12,800 consecutive patients from 64 hospitals in seven low-middle income countries. Eight strategies to minimise and monitor bias were pre-specified: (1) minimum of 4 hospitals per country; (2) pre-randomisation identification of units of exposure (operating theatres, lists, teams or sessions) within clusters; (3) minimisation of randomisation by country and hospital type; (4) site training delivered after randomisation; (5) dedicated 'warm-up week' to train teams; (6) trial specific sticker and patient register to monitor consecutive patient identification; (7) monitoring characteristics of patients and units of exposure; and (8) low-burden outcome-assessment.
Results: This analysis includes 10,686 patients from 70 clusters. The results aligned to the eight strategies were (1) 6 out of 7 countries included ≥ 4 hospitals; (2) 87.1% (61/70) of hospitals maintained their planned operating theatres (82% [27/33] and 92% [34/37] in the intervention and control arms); (3) minimisation maintained balance of key factors in both arms; (4) post-randomisation training was conducted for all hospitals; (5) the 'warm-up week' was conducted at all sites, and feedback used to refine processes; (6) the sticker and trial register were maintained, with an overall inclusion of 98.1% (10,686/10,894) of eligible patients; (7) monitoring allowed swift identification of problems in patient inclusion and key patient characteristics were reported: malignancy (20.3% intervention vs 12.6% control), midline incisions (68.4% vs 58.9%) and elective surgery (52.4% vs 42.6%); and (8) 0.4% (41/9187) of patients refused consent for outcome assessment.
Conclusion: cRCTs in surgery have several potential sources of bias that include varying units of exposure and the need for consecutive inclusion of all eligible patients across complex settings. We report a system that monitored and minimised the risks of bias and imbalances by arm, with important lessons for future cRCTs within hospitals.
Keywords: Abdominal surgery; Bias; Cluster randomised controlled trial; Global health; Global surgery; Quality assurance; Research methodology; Surgical site infection; Trial management.
© 2023. The Author(s).
Conflict of interest statement
The author declares that there are no competing interests.
Figures
References
-
- Collaborative EESA. ESCP Safe Anastomosis ProGramme in CoLorectal SurgEry (EAGLE): study protocol for an international cluster randomised trial of a quality improvement intervention to reduce anastomotic leak following right colectomy. Colorectal Dis. 2021;23(10):2761–2771. doi: 10.1111/codi.15806. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources