Optimization of guanosine-based hydrogels with boric acid derivatives for enhanced long-term stability and cell survival
- PMID: 37020512
- PMCID: PMC10069680
- DOI: 10.3389/fbioe.2023.1147943
Optimization of guanosine-based hydrogels with boric acid derivatives for enhanced long-term stability and cell survival
Abstract
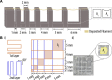
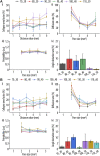
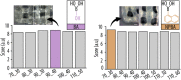
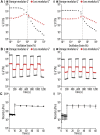
Tissue defects can lead to serious health problems and often require grafts or transplants to repair damaged soft tissues. However, these procedures can be complex and may not always be feasible due to a lack of available tissue. Hydrogels have shown potential as a replacement for tissue grafts due to their ability to support cell survival and encapsulate biomolecules such as growth factors. In particular, guanosine-based hydrogels have been explored as a potential solution, but they often exhibit limited stability which hampers their use in the biofabrication of complex grafts. To address this issue, we explored the use of borate ester chemistry and more complex boric acid derivatives to improve the stability and properties of guanosine-based hydrogels. We hypothesized that the aromatic rings in these derivatives would enhance the stability and printability of the hydrogels through added π-π stack interactions. After optimization, 13 compositions containing either 2-naphthylboronic acid or boric acid were selected. Morphology studies shows a well-defined nanofibrilar structure with good printable properties (thixotropic behaviour, print fidelity and printability). Moreover, the pH of all tested hydrogels was within the range suitable for cell viability (7.4-8.3). Nevertheless, only the boric acid-based formulations were stable for at least 7 days. Thus, our results clearly demonstrated that the presence of additional aromatic rings did actually impair the hydrogel properties. We speculate that this is due to steric hindrance caused by adjacent groups, which disrupt the correct orientation of the aromatic groups required for effective π-π stack interactions of the guanosine building block. Despite this drawback, the developed guanosine-boric acid hydrogel exhibited good thixotropic properties and was able to support cell survival, proliferation, and migration. For instance, SaOS-2 cells planted on these printed structures readily migrated into the hydrogel and showed nearly 100% cell viability after 7 days. In conclusion, our findings highlight the potential of guanosine-boric acid hydrogels as tissue engineering scaffolds that can be readily enhanced with living cells and bioactive molecules. Thus, our work represents a significant advancement towards the development of functionalized guanosine-based hydrogels.
Keywords: 3D printing; boric acid derivatives; guanosine-based hydrogels; nucleoside; printable hydrogels.
Copyright © 2023 Merino-Gómez, Godoy-Gallardo, Wendner, Mateos-Timoneda, Gil and Perez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Polydopamine Incorporation Enhances Cell Differentiation and Antibacterial Properties of 3D-Printed Guanosine-Borate Hydrogels for Functional Tissue Regeneration.Int J Mol Sci. 2023 Feb 20;24(4):4224. doi: 10.3390/ijms24044224. Int J Mol Sci. 2023. PMID: 36835636 Free PMC article.
-
Advanced Binary Guanosine and Guanosine 5'-Monophosphate Cell-Laden Hydrogels for Soft Tissue Reconstruction by 3D Bioprinting.ACS Appl Mater Interfaces. 2023 Jun 28;15(25):29729-29742. doi: 10.1021/acsami.2c23277. Epub 2023 Jun 15. ACS Appl Mater Interfaces. 2023. PMID: 37319328
-
Nucleoside-Based Supramolecular Hydrogels: From Synthesis and Structural Properties to Biomedical and Tissue Engineering Applications.ACS Biomater Sci Eng. 2023 Jan 9;9(1):40-61. doi: 10.1021/acsbiomaterials.2c01051. Epub 2022 Dec 16. ACS Biomater Sci Eng. 2023. PMID: 36524860 Review.
-
Advancing bioinks for 3D bioprinting using reactive fillers: A review.Acta Biomater. 2020 Sep 1;113:1-22. doi: 10.1016/j.actbio.2020.06.040. Epub 2020 Jul 2. Acta Biomater. 2020. PMID: 32622053 Review.
-
Three-Dimensional-Printable Thermo/Photo-Cross-Linked Methacrylated Chitosan-Gelatin Hydrogel Composites for Tissue Engineering.ACS Appl Mater Interfaces. 2021 May 19;13(19):22902-22913. doi: 10.1021/acsami.1c01321. Epub 2021 May 7. ACS Appl Mater Interfaces. 2021. PMID: 33960765
Cited by
-
Dancing with Nucleobases: Unveiling the Self-Assembly Properties of DNA and RNA Base-Containing Molecules for Gel Formation.Gels. 2023 Dec 23;10(1):16. doi: 10.3390/gels10010016. Gels. 2023. PMID: 38247739 Free PMC article. Review.
-
Advanced optical assessment and modeling of extrusion bioprinting.Sci Rep. 2024 Jun 17;14(1):13972. doi: 10.1038/s41598-024-64039-y. Sci Rep. 2024. PMID: 38886452 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

