Case report: CAR-T cell therapy-induced cardiac tamponade
- PMID: 37020516
- PMCID: PMC10067676
- DOI: 10.3389/fcvm.2023.1132503
Case report: CAR-T cell therapy-induced cardiac tamponade
Abstract
CD19-specific chimeric antigen receptor T (CAR-T) cell therapy has recently been shown to improve the prognosis of refractory diffuse large B-cell lymphoma (DLBCL). However, CAR-T cells may induce numerous adverse events, in particular cytokine release syndrome (CRS) which is frequently associated with cardiovascular manifestations. Among the latter, acute pericardial effusion represents less than 1% of cases and cardiac tamponade has only been reported once. The management and outcome of these severe complications are not well established. We report here, a case of cardiac tamponade associated with CRS in a context of CAR-T cell therapy, which required urgent pericardiocentesis.
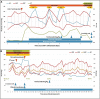
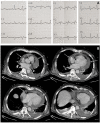
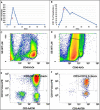
Case summary: A 65-year-old man with refractory DLBCL was treated with CAR-T cell therapy. He had a history of dilated cardiomyopathy with preserved ejection fraction and transient atrial fibrillation. A pericardial localization of the lymphoma was observed on the second relapse. One day after CAR-T cell infusion the patient was diagnosed with grade 1 CRS. Due to hypotension, he was treated with tocilizumab and dexamethasone, and then transferred to intensive care unit (ICU). Echocardiography performed at ICU admission showed acute pericardial effusion with signs of right ventricular heart failure due to cardiac tamponade. It was decided to perform pericardiocentesis despite grade IV thrombocytopenia in a context of aplasia. Analysis of pericardial fluid showed a large number of lymphoma cells and 73% of CAR-T cells amongst lymphocytes, a level that was similar in blood. Hemodynamic status improved after pericardiocentesis, and no recurrence of pericardial effusion was observed. The presence of a high count of activated CAR-T cells in the pericardial fluid as well as the short interval between CAR-T cells injection and the symptoms appear as potential arguments for a direct action of CAR-T cells in the mechanism of this adverse event. The patient was discharged from ICU after two days and initially exhibited a good response to DLBCL treatment. Unfortunately, he died fifty days after starting CAR-T cell therapy due to a new DLBCL relapse.
Conclusion: Patients with a pericardial localization of DLBCL should be assessed for a risk of cardiac tamponade if receiving CAR-T cell therapy and presenting CRS. In this case, cardiac tamponade seems directly related to CAR-T cell expansion. Pericardiocentesis should be considered as a feasible and effective treatment if the risk of bleeding is well controlled, in association with anti-IL6 and corticosteroids.
Keywords: CAR-T cell; CRS; ICU; cardiac tamponade; case report; pericardial effusion; pericarditis.
© 2023 Sarfati, Norbert, Hérault, Giry, Makké, Grall, Savouré, Camus, Alani, Tamion, Latouche and Girault.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Case Report: Cardiac Tamponade in Association With Cytokine Release Syndrome Following CAR-T Cell Therapy.Front Cardiovasc Med. 2022 Mar 21;9:848091. doi: 10.3389/fcvm.2022.848091. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35387436 Free PMC article.
-
CD19 and CD70 Dual-Target Chimeric Antigen Receptor T-Cell Therapy for the Treatment of Relapsed and Refractory Primary Central Nervous System Diffuse Large B-Cell Lymphoma.Front Oncol. 2019 Dec 4;9:1350. doi: 10.3389/fonc.2019.01350. eCollection 2019. Front Oncol. 2019. PMID: 31867275 Free PMC article.
-
Cervical Local Cytokine Release Syndrome Following Chimeric Antigen Receptor T-cell Therapy in Patients With Relapsed or Refractory Diffuse Large B-cell Lymphoma.Cureus. 2023 May 11;15(5):e38905. doi: 10.7759/cureus.38905. eCollection 2023 May. Cureus. 2023. PMID: 37303362 Free PMC article.
-
Pericardial tamponade: A comprehensive emergency medicine and echocardiography review.Am J Emerg Med. 2022 Aug;58:159-174. doi: 10.1016/j.ajem.2022.05.001. Epub 2022 May 6. Am J Emerg Med. 2022. PMID: 35696801 Review.
-
Double systemic cytokine release syndrome following sequential infusion of anti-CD22 and anti-CD19 chimeric antigen receptor T cells after autologous hematopoietic stem cell transplantation for a central diffuse large B-cell lymphoma patient: A case report and literature review.Front Immunol. 2023 Jan 31;14:1098815. doi: 10.3389/fimmu.2023.1098815. eCollection 2023. Front Immunol. 2023. PMID: 36798130 Free PMC article. Review.
Cited by
-
Epcoritamab-Induced fatal pleural effusion in diffuse large B-Cell lymphoma: a case report and literature review.Ann Hematol. 2025 Mar;104(3):1995-2000. doi: 10.1007/s00277-025-06206-3. Epub 2025 Jan 18. Ann Hematol. 2025. PMID: 39825138 Free PMC article. Review.
-
Influence of CAR T-cell therapy associated complications.Front Oncol. 2025 Feb 20;15:1494986. doi: 10.3389/fonc.2025.1494986. eCollection 2025. Front Oncol. 2025. PMID: 40052127 Free PMC article. Review.
-
Systemic toxicity of CAR-T therapy and potential monitoring indicators for toxicity prevention.Front Immunol. 2024 Aug 26;15:1422591. doi: 10.3389/fimmu.2024.1422591. eCollection 2024. Front Immunol. 2024. PMID: 39253080 Free PMC article. Review.
References
-
- Westin JR, Kersten MJ, Salles G, Abramson JS, Schuster SJ, Locke FL, et al. Efficacy and safety of CD19-directed CAR-T cell therapies in patients with relapsed/refractory aggressive B-cell lymphomas: observations from the JULIET, ZUMA-1, and TRANSCEND trials. Am J Hematol. (2021) 96(10):1295–312. 10.1002/ajh.26301 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

