Case report: Preliminary response to tislelizumab plus S-1 in patients with metastatic gallbladder carcinoma: A report of five cases and a literature review
- PMID: 37020545
- PMCID: PMC10067585
- DOI: 10.3389/fimmu.2023.1144371
Case report: Preliminary response to tislelizumab plus S-1 in patients with metastatic gallbladder carcinoma: A report of five cases and a literature review
Abstract
Gallbladder cancer (GBC) and cholangiocarcinoma are common cancers of the biliary system and are associated with a poor prognosis. Surgery and chemotherapy provide limited benefit to patients with advanced biliary tract carcinoma. Novel immunotherapies and molecularly targeted therapies are more effective options; however, few patients benefit and drug resistance is a concern. Here, we report five cases of advanced GBC with either high programmed death-ligand 1 (PD-L1) expression or a high tumor mutation burden (TMB-H). The patients were treated with a combination therapy of tislelizumab and S-1. The tumors were effectively controlled in most patients. One patient developed immune-related pneumonia (irP) during treatment, which resolved after hormone therapy, and the patient underwent surgery. Tislelizumab and S-1 were administered again after surgery; however, recurrent irP required discontinuation, and the tumor progressed after drug withdrawal. These cases demonstrate that combined therapy of anti-programmed cell death protein-1 (PD-1) antibodies and S-1 is a safe and effective regimen with few side effects for GBC patients, especially for sensitive populations (patients with TMB-H, microsatellite instability, deficient mismatch repair, or high expression of PD-L1). To our knowledge, this is the first time that tislelizumab in combination with S-1 has been used to treat patients with advanced GBC.
Keywords: GBC; PD-L1; S-1; immunotharapy; tislelizumab.
Copyright © 2023 Zhang, Liu, Liu, Liu, Xiong, Li, Fu, Zhou, Liao, Fang and Liang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
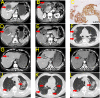
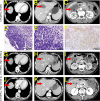
Figures


Similar articles
-
Molecular Characterization of Biliary Tract Cancer Predicts Chemotherapy and Programmed Death 1/Programmed Death-Ligand 1 Blockade Responses.Hepatology. 2021 Oct;74(4):1914-1931. doi: 10.1002/hep.31862. Epub 2021 Jul 29. Hepatology. 2021. PMID: 33884649
-
Local and abscopal responses in advanced intrahepatic cholangiocarcinoma with low TMB, MSS, pMMR and negative PD-L1 expression following combined therapy of SBRT with PD-1 blockade.J Immunother Cancer. 2019 Aug 5;7(1):204. doi: 10.1186/s40425-019-0692-z. J Immunother Cancer. 2019. PMID: 31383016 Free PMC article.
-
Significant response to anti-PD-1 based immunotherapy plus lenvatinib for recurrent intrahepatic cholangiocarcinoma with bone metastasis: A case report and literature review.Medicine (Baltimore). 2019 Nov;98(45):e17832. doi: 10.1097/MD.0000000000017832. Medicine (Baltimore). 2019. PMID: 31702638 Free PMC article. Review.
-
Programmed Death Ligand 1 Expression as a Prognostic Marker in Patients with Advanced Biliary Tract Cancer.Oncology. 2021;99(6):365-372. doi: 10.1159/000514404. Epub 2021 Mar 17. Oncology. 2021. PMID: 33730723
-
A metastatic intrahepatic cholangiocarcinoma treated with programmed cell death 1 inhibitor: a case report and literature review.Immunotherapy. 2020 Jun;12(8):555-561. doi: 10.2217/imt-2019-0100. Epub 2020 May 6. Immunotherapy. 2020. PMID: 32372672 Review.
Cited by
-
Research progress on prognostic factors of gallbladder carcinoma.J Cancer Res Clin Oncol. 2024 Oct 6;150(10):447. doi: 10.1007/s00432-024-05975-0. J Cancer Res Clin Oncol. 2024. PMID: 39369366 Free PMC article. Review.
-
Exosomes derived from umbilical cord blood NK cells inhibit the progression of pancreatic cancer by targeting ROS-mediated mitochondrial dysfunction.Saudi Pharm J. 2025 Apr 29;33(1-2):8. doi: 10.1007/s44446-025-00009-3. Saudi Pharm J. 2025. PMID: 40397293 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

