Protection from COVID-19 disease in hamsters vaccinated with subunit SARS-CoV-2 S1 mucosal vaccines adjuvanted with different adjuvants
- PMID: 37020550
- PMCID: PMC10067881
- DOI: 10.3389/fimmu.2023.1154496
Protection from COVID-19 disease in hamsters vaccinated with subunit SARS-CoV-2 S1 mucosal vaccines adjuvanted with different adjuvants
Abstract
Introduction: Adjuvant plays an important role in directing the immune responses induced by vaccines. In previous studies, we have shown that a mucosal SARS-CoV-2 S1 subunit vaccine adjuvanted with a combination of CpG, Poly I:C and IL-15 (named CP15) induced effective mucosal and systemic immunity and conferred nearly sterile protection against SARS-CoV-2 viral replication in macaque models.
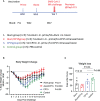
Methods: In this study, we used a hamster model, which mimics the human scenario and reliably exhibits severe SARS-CoV-2 disease similar to hospitalized patients, to investigate the protection efficacy of the vaccines against COVID-19 disease. We compared the weight loss, viral loads (VLs), and clinical observation scores of three different vaccine regimens. All three regimens consisted of priming/boosting with S1 subunit vaccines, but adjuvanted with alum and/or CP15 administrated by either intramuscular (IM) or intranasal (IN) routes: Group 1 was adjuvanted with alum/alum administrated IM/IM; Group 2 was alum-IM/CP15-IN; and Group 3 was CP15-IM/CP15-IN.
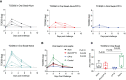
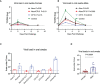
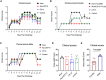
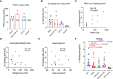
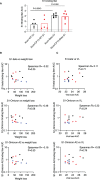
Results: After challenge with SARS-CoV-2 WA strain, we found that the alum/CP15 group showed best protection against weight loss, while the CP15 group demonstrated best reduction of oral SARS-CoV-2 VLs, suggesting that the protection profiles were different. Sex differences for VL and clinical scores were observed. Humoral immunity was induced but not correlated with protection. Moreover, S1-specific binding antibody titers against beta, omicron BA.1, and BA.2 variants showed 2.6-, 4.9- and 2.8- fold reduction, respectively, compared to the Wuhan strain.
Discussion: Overall, the data suggested that adjuvants in subunit vaccines determine the protection profiles after SARS-CoV-2 infection and that nasal/oral mucosal immunization can protect against systemic COVID-19 disease.
Keywords: COVID-19; SARS-CoV-2; hamster model; intranasal vaccine; mucosal adjuvant.
Copyright © 2023 Sui, Andersen, Li, Hoang, Bekele, Kar, Lewis and Berzofsky.
Conflict of interest statement
Authors HA, SK, and ML are employed by Bioqual Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Adjuvanted subunit intranasal vaccine reduces SARS-CoV-2 onward transmission in hamsters.Front Immunol. 2025 Feb 7;16:1514845. doi: 10.3389/fimmu.2025.1514845. eCollection 2025. Front Immunol. 2025. PMID: 39981227 Free PMC article.
-
Intranasal nanoemulsion adjuvanted S-2P vaccine demonstrates protection in hamsters and induces systemic, cell-mediated and mucosal immunity in mice.PLoS One. 2022 Nov 2;17(11):e0272594. doi: 10.1371/journal.pone.0272594. eCollection 2022. PLoS One. 2022. PMID: 36322572 Free PMC article.
-
Intranasal administration of unadjuvanted SARS-CoV-2 spike antigen boosts antigen-specific immune responses induced by parenteral protein subunit vaccine prime in mice and hamsters.Eur J Immunol. 2024 Jun;54(6):e2350620. doi: 10.1002/eji.202350620. Epub 2024 Apr 1. Eur J Immunol. 2024. PMID: 38561974
-
Intranasal COVID-19 vaccines: From bench to bed.EBioMedicine. 2022 Feb;76:103841. doi: 10.1016/j.ebiom.2022.103841. Epub 2022 Jan 24. EBioMedicine. 2022. PMID: 35085851 Free PMC article. Review.
-
Nasal vaccination against SARS-CoV-2: Synergistic or alternative to intramuscular vaccines?Int J Pharm. 2021 Jun 15;603:120686. doi: 10.1016/j.ijpharm.2021.120686. Epub 2021 May 6. Int J Pharm. 2021. PMID: 33964339 Free PMC article. Review.
Cited by
-
Adjuvanted subunit intranasal vaccine reduces SARS-CoV-2 onward transmission in hamsters.Front Immunol. 2025 Feb 7;16:1514845. doi: 10.3389/fimmu.2025.1514845. eCollection 2025. Front Immunol. 2025. PMID: 39981227 Free PMC article.
-
Sex-biased immunogenicity of a mucosal subunit vaccine against SARS-CoV-2 in mice.Front Immunol. 2024 May 21;15:1386243. doi: 10.3389/fimmu.2024.1386243. eCollection 2024. Front Immunol. 2024. PMID: 38835757 Free PMC article.
-
The immunobiology of SARS-CoV-2 infection and vaccine responses: potential influences of cross-reactive memory responses and aging on efficacy and off-target effects.Front Immunol. 2024 Feb 26;15:1345499. doi: 10.3389/fimmu.2024.1345499. eCollection 2024. Front Immunol. 2024. PMID: 38469293 Free PMC article. Review.
-
SARS-CoV-2 mucosal vaccine protects against clinical disease with sex bias in efficacy.Vaccine. 2024 Jan 12;42(2):339-351. doi: 10.1016/j.vaccine.2023.11.059. Epub 2023 Dec 8. Vaccine. 2024. PMID: 38071106 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

