Food and Drug Administration Guidance on Design of Clinical Trials for Gene Therapy Products with Potential for Genome Integration or Genome Editing and Associated Long-Term Follow-Up of Research Subjects
- PMID: 37020567
- PMCID: PMC10068672
- DOI: 10.1089/apb.2022.0022
Food and Drug Administration Guidance on Design of Clinical Trials for Gene Therapy Products with Potential for Genome Integration or Genome Editing and Associated Long-Term Follow-Up of Research Subjects
Abstract
Introduction: With the burgeoning growth of the gene therapy industry, the Food and Drug Administration (FDA) has produced various guidance documents intended to help gene therapy manufacturers design their preclinical testing and clinical trials to facilitate the process of obtaining marketing approval.
Discussion: Biosafety professionals and institutional biosafety committees (IBCs) with oversight of clinical trials or biopharmaceutical manufacturing stand to benefit from understanding how these guidance documents set the standard for writing the clinical research protocols that are reviewed by IBCs. Although the FDA guidance documents are typically meant for manufacturers (either pharmaceutical companies serving as research sponsors or investigators at academic institutions), much of the content is useful for biosafety professionals and IBCs during the IBC review process.
Conclusion: This article specifically addresses guidance documents pertaining to gene therapy vectors capable of genomic integration, testing for replication competent retrovirus, genome editing, and long-term follow-up of research subjects.
Keywords: FDA guidance; clinical trials; gene editing; gene therapy; long-term follow-up; replication competent retrovirus.
Copyright 2022, ABSA International 2022.
Conflict of interest statement
The authors are employed by Advarra, a for-profit entity providing independent IBC and IRB reviews.
Figures
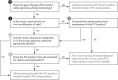
References
-
- Eisenman D. The United States' regulatory environment is evolving to accommodate a coming boom in gene therapy research. Appl Biosaf 2019;24(3):147–152; doi: org/10.1177/1535676019854866. - PMC - PubMed
-
- Eisenman D, Debold S, Riddle J. A changing world in gene therapy research: Exciting opportunities for medical advancement and biosafety challenges. Appl Biosaf 2021;26(4):179–192; doi: org/10.1089/apb.2021.0020. - PMC - PubMed
-
- Stolberg SG. The biotech death of Jesse Gelsinger. New York Times Magazine, Nov 28, 1999, Section 6: 137–150. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
