Autophagy and its therapeutic potential in diabetic nephropathy
- PMID: 37020591
- PMCID: PMC10067862
- DOI: 10.3389/fendo.2023.1139444
Autophagy and its therapeutic potential in diabetic nephropathy
Abstract
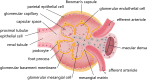
Diabetic nephropathy (DN), the leading cause of end-stage renal disease, is the most significant microvascular complication of diabetes and poses a severe public health concern due to a lack of effective clinical treatments. Autophagy is a lysosomal process that degrades damaged proteins and organelles to preserve cellular homeostasis. Emerging studies have shown that disorder in autophagy results in the accumulation of damaged proteins and organelles in diabetic renal cells and promotes the development of DN. Autophagy is regulated by nutrient-sensing pathways including AMPK, mTOR, and Sirt1, and several intracellular stress signaling pathways such as oxidative stress and endoplasmic reticulum stress. An abnormal nutritional status and excess cellular stresses caused by diabetes-related metabolic disorders disturb the autophagic flux, leading to cellular dysfunction and DN. Here, we summarized the role of autophagy in DN focusing on signaling pathways to modulate autophagy and therapeutic interferences of autophagy in DN.
Keywords: autophagy; cellular stress; diabetic nephropathy; nutrient-sensing pathway; renal cell.
Copyright © 2023 Han, Liu, Yan, Chen, Meng, Zhou and Qian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

