Modeling and Thermodynamic Studies of γ-Valerolactone Production from Bio-derived Methyl Levulinate
- PMID: 37020618
- PMCID: PMC10069308
- DOI: 10.1002/gch2.202200208
Modeling and Thermodynamic Studies of γ-Valerolactone Production from Bio-derived Methyl Levulinate
Abstract
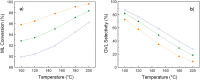
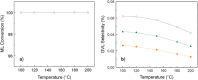
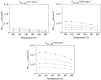
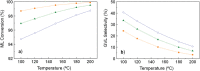
The exploitation of biomass to reduce the dependency on fossil fuels represents a challenge that needs to be solved as soon as possible. Nowadays, one of the most fashionable processes is γ-valerolactone (GVL) production from bio-derived methyl levulinate (ML). Deep understanding of the thermodynamic aspects involved in this process is key for a successful outcome, but detailed studies are missing in the existing literature. A thermodynamic study of the reaction of γ-valerolactone (GVL) production from bio-derived methyl levulinate (ML) is performed by the Gibbs free energy minimization method. The effect of various reaction conditions (temperature, concentration, flow rate) and the implication of possible intermediates and byproducts are assessed. Conversion and selectivity are calculated from the simulation of the ML hydrogenation using isopropanol as the hydrogen donor under continuous flow conditions. Significant increases in GVL selectivity can be achieved under dry conditions, keeping the high conversion. Comparison between theoretical and experimental results from a previous article discloses the effect of using 5%RuTiO2 catalysts, which increases the selectivity from 3-40% to 41-98%. Enthalpy and Gibbs free energy of the reactions at issue are also calculated from models using Barin equations according to Aspen Physical Property System parameters.
Keywords: Aspen Plus; Hydrogenation; Methyl levulinate; Thermodynamic analysis; γ‐valerolactone.
© 2023 The Authors. Global Challenges published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Stöcker M., Angew. Chem., Int. Ed. 2008, 47, 9200. - PubMed
-
- Alonso D. M., Bond J. Q., Dumesic J. A., Green Chem. 2010, 12, 1493.
-
- Alonso D. M., Wettstein S. G., Dumesic J. A., Chem. Soc. Rev. 2012, 41, 8075. - PubMed
-
- García A., Miguel P. J., Pico M. P., Álvarez‐Serrano I., López M. L., García T., Solsona B., Appl. Catal. Gen. 2021, 623, 118276.
-
- Huber G. W., Corma A., Angew. Chem., Int. Ed. 2007, 46, 7184. - PubMed
LinkOut - more resources
Full Text Sources

