(2R,6R)-Hydroxynorketamine Treatment of Rats Exposed to Repetitive Low-Level Blast Injury
- PMID: 37020715
- PMCID: PMC10068674
- DOI: 10.1089/neur.2022.0088
(2R,6R)-Hydroxynorketamine Treatment of Rats Exposed to Repetitive Low-Level Blast Injury
Abstract
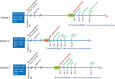
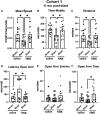
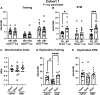
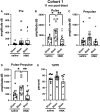
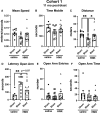
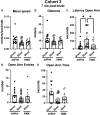
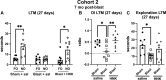
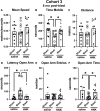
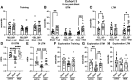
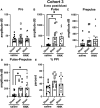
Many military veterans who experienced blast-related traumatic brain injuries (TBIs) in the conflicts in Iraq and Afghanistan suffer from chronic cognitive and mental health problems, including post-traumatic stress disorder (PTSD). Male rats subjected to repetitive low-level blast exposure develop chronic cognitive and PTSD-related traits that develop in a delayed manner. Ketamine has received attention as a treatment for refractory depression and PTSD. (2R,6R)-hydroxynorketamine [(2R,6R)-HNK] is a ketamine metabolite that exerts rapid antidepressant actions. (2R,6R)-HNK has become of clinical interest because of its favorable side-effect profile, low abuse potential, and oral route of administration. We treated three cohorts of blast-exposed rats with (2R,6R)-HNK, beginning 7-11 months after blast exposure, a time when the behavioral phenotype is established. Each cohort consisted of groups (n = 10-13/group) as follows: 1) Sham-exposed treated with saline, 2) blast-exposed treated with saline, and 3) blast-exposed treated with a single dose of 20 mg/kg of (2R,6R)-HNK. (2R,6R)-HNK rescued blast-induced deficits in novel object recognition (NOR) and anxiety-related features in the elevated zero maze (EZM) in all three cohorts. Exaggerated acoustic startle was reversed in cohort 1, but not in cohort 3. (2R,6R)-HNK effects were still present in the EZM 12 days after administration in cohort 1 and 27 days after administration in NOR testing of cohorts 2 and 3. (2R,6R)-HNK may be beneficial for the neurobehavioral syndromes that follow blast exposure in military veterans. Additional studies will be needed to determine whether higher doses or more extended treatment regimens may be more effective.
Keywords: (2R,6R)-hydroxynorketamine; blast; post-traumatic stress disorder; rat; traumatic brain injury.
© Georgina Perez Garcia et al., 2023; Published by Mary Ann Liebert, Inc.
Conflict of interest statement
No competing financial interests exist.
Figures


Similar articles
-
Ketamine metabolite (2R,6R)-hydroxynorketamine reverses behavioral despair produced by adolescent trauma.Pharmacol Biochem Behav. 2020 Sep;196:172973. doi: 10.1016/j.pbb.2020.172973. Epub 2020 Jun 20. Pharmacol Biochem Behav. 2020. PMID: 32569786 Free PMC article.
-
(2R,6R)-hydroxynorketamine acts through GluA1-induced synaptic plasticity to alleviate PTSD-like effects in rat models.Neurobiol Stress. 2022 Nov 28;21:100503. doi: 10.1016/j.ynstr.2022.100503. eCollection 2022 Nov. Neurobiol Stress. 2022. PMID: 36532380 Free PMC article.
-
(2R,6R)-hydroxynorketamine exerts mGlu2 receptor-dependent antidepressant actions.Proc Natl Acad Sci U S A. 2019 Mar 26;116(13):6441-6450. doi: 10.1073/pnas.1819540116. Epub 2019 Mar 13. Proc Natl Acad Sci U S A. 2019. PMID: 30867285 Free PMC article.
-
The antidepressant potential of (2R,6R)-hydroxynorketamine: A detailed review of pre-clinical findings.Eur J Pharmacol. 2025 Jul 15;999:177604. doi: 10.1016/j.ejphar.2025.177604. Epub 2025 Apr 8. Eur J Pharmacol. 2025. PMID: 40209847 Review.
-
Hydroxynorketamines: Pharmacology and Potential Therapeutic Applications.Pharmacol Rev. 2021 Apr;73(2):763-791. doi: 10.1124/pharmrev.120.000149. Pharmacol Rev. 2021. PMID: 33674359 Free PMC article. Review.
Cited by
-
The Neurovascular Unit as a Locus of Injury in Low-Level Blast-Induced Neurotrauma.Int J Mol Sci. 2024 Jan 17;25(2):1150. doi: 10.3390/ijms25021150. Int J Mol Sci. 2024. PMID: 38256223 Free PMC article. Review.
-
Serotonin 5-HT2A receptor expression is chronically decreased in the anterior cerebral cortex of male rats following repetitive low-level blast exposure.Front Neurol. 2025 Jun 25;16:1594335. doi: 10.3389/fneur.2025.1594335. eCollection 2025. Front Neurol. 2025. PMID: 40635716 Free PMC article.
References
-
- Hoge CW, McGurk D, Thomas JL, et al. . Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N Engl J Med 2008;358(5):453–63 - PubMed
-
- Tanielian T, Jaycox LH, (eds). Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. Rand Corporation: Santa Monica, CA; 2008.
-
- Engel C, Hoch E, Simmons M.. The Neurological Effects of Repeated Exposure to Military Occupational Blast: Implications for Prevention and Health: Proceedings, Findings, and Expert Recommendations from the Seventh Department of Defense State-of-the-Science Meeting. Rand Corporation: Arlington, VA; 2019.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
