Brain vitamin D3-auto/paracrine system in relation to structural, neurophysiological, and behavioral disturbances associated with glucocorticoid-induced neurotoxicity
- PMID: 37020845
- PMCID: PMC10067932
- DOI: 10.3389/fncel.2023.1133400
Brain vitamin D3-auto/paracrine system in relation to structural, neurophysiological, and behavioral disturbances associated with glucocorticoid-induced neurotoxicity
Abstract
Introduction: Vitamin D3 (VD3) is a potent para/autocrine regulator and neurosteroid that can strongly influence nerve cell function and counteract the negative effects of glucocorticoid (GC) therapy. The aim of the study was to reveal the relationship between VD3 status and behavioral, structural-functional and molecular changes associated with GC-induced neurotoxicity.
Methods: Female Wistar rats received synthetic GC prednisolone (5 mg/kg b.w.) with or without VD3 (1000 IU/kg b.w.) for 30 days. Behavioral, histological, physiological, biochemical, molecular biological (RT-PCR, Western blotting) methods, and ELISA were used.
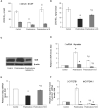
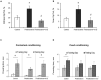
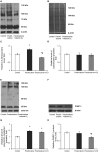
Results and discussion: There was no difference in open field test (OFT), while forced swim test (FST) showed an increase in immobility time and a decrease in active behavior in prednisolone-treated rats, indicative of depressive changes. GC increased the perikaryon area, enlarged the size of the nuclei, and caused a slight reduction of cell density in CA1-CA3 hippocampal sections. We established a GC-induced decrease in the long-term potentiation (LTP) in CA1-CA3 hippocampal synapses, the amplitude of high K+-stimulated exocytosis, and the rate of Ca2+-dependent fusion of synaptic vesicles with synaptic plasma membranes. These changes were accompanied by an increase in nitration and poly(ADP)-ribosylation of cerebral proteins, suggesting the development of oxidative-nitrosative stress. Prednisolone upregulated the expression and phosphorylation of NF-κB p65 subunit at Ser311, whereas downregulating IκB. GC loading depleted the circulating pool of 25OHD3 in serum and CSF, elevated VDR mRNA and protein levels but had an inhibitory effect on CYP24A1 and VDBP expression. Vitamin D3 supplementation had an antidepressant-like effect, decreasing the immobility time and stimulating active behavior. VD3 caused a decrease in the size of the perikaryon and nucleus in CA1 hippocampal area. We found a recovery in depolarization-induced fusion of synaptic vesicles and long-term synaptic plasticity after VD3 treatment. VD3 diminished the intensity of oxidative-nitrosative stress, and suppressed the NF-κB activation. Its ameliorative effect on GC-induced neuroanatomical and behavioral abnormalities was accompanied by the 25OHD3 repletion and partial restoration of the VD3-auto/paracrine system.
Conclusion: GC-induced neurotoxicity and behavioral disturbances are associated with increased oxidative-nitrosative stress and impairments of VD3 metabolism. Thus, VD3 can be effective in preventing structural and functional abnormalities in the brain and behavior changes caused by long-term GC administration.
Keywords: behavioral impairments; glucocorticoid-induced neurotoxicity; glucocorticoids; oxidative-nitrosative stress; prednisolone; vitamin D-auto/paracrine system; vitamin D3.
Copyright © 2023 Lisakovska, Labudzynskyi, Khomenko, Isaev, Savotchenko, Kasatkina, Savosko, Veliky and Shymanskyi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Vitamin D Auto-/Paracrine System Is Involved in Modulation of Glucocorticoid-Induced Changes in Angiogenesis/Bone Remodeling Coupling.Int J Endocrinol. 2020 Sep 4;2020:8237610. doi: 10.1155/2020/8237610. eCollection 2020. Int J Endocrinol. 2020. PMID: 32952554 Free PMC article.
-
Vitamin D3 Modulates Impaired Crosstalk Between RANK and Glucocorticoid Receptor Signaling in Bone Marrow Cells After Chronic Prednisolone Administration.Front Endocrinol (Lausanne). 2018 Jun 7;9:303. doi: 10.3389/fendo.2018.00303. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29930537 Free PMC article.
-
Vitamin D3 protects against prednisolone-induced liver injury associated with the impairment of the hepatic NF-κB/iNOS/NO pathway.Biochem Cell Biol. 2017 Apr;95(2):213-222. doi: 10.1139/bcb-2016-0070. Epub 2016 Jul 14. Biochem Cell Biol. 2017. PMID: 28004974
-
1alpha(OH)D3 One-alpha-hydroxy-cholecalciferol--an active vitamin D analog. Clinical studies on prophylaxis and treatment of secondary hyperparathyroidism in uremic patients on chronic dialysis.Dan Med Bull. 2008 Nov;55(4):186-210. Dan Med Bull. 2008. PMID: 19232159 Review.
-
The burgeoning role of cytochrome P450-mediated vitamin D metabolites against colorectal cancer.Pharmacol Res. 2018 Jul;133:9-20. doi: 10.1016/j.phrs.2018.04.022. Epub 2018 Apr 30. Pharmacol Res. 2018. PMID: 29719203 Review.
Cited by
-
Investigating the Impact of Selective Modulators on the Renin-Angiotensin-Aldosterone System: Unraveling Their Off-Target Perturbations of Transmembrane Ionic Currents.Int J Mol Sci. 2023 Sep 12;24(18):14007. doi: 10.3390/ijms241814007. Int J Mol Sci. 2023. PMID: 37762309 Free PMC article. Review.
-
Functional Food Nutrients, Redox Resilience Signaling and Neurosteroids for Brain Health.Int J Mol Sci. 2024 Nov 12;25(22):12155. doi: 10.3390/ijms252212155. Int J Mol Sci. 2024. PMID: 39596221 Free PMC article. Review.
References
-
- Alan I. S., Alan B. (2017). “Side effects of glucocorticoids,” in Pharmacokinetics and adverse effects of drugs–mechanisms and risks factors, ed. Malangu N. (London: IntechOpen; ). 10.5772/intechopen.72019 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

