Specific sequence mutations in a long-lasting rhIFN-α2b version reduce in vitro and in vivo immunogenicity and increase in vitro protein stability
- PMID: 37020947
- PMCID: PMC10068115
- DOI: 10.1016/j.heliyon.2023.e14670
Specific sequence mutations in a long-lasting rhIFN-α2b version reduce in vitro and in vivo immunogenicity and increase in vitro protein stability
Abstract
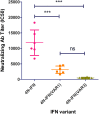
For decades, recombinant human interferon alpha (rhIFN-α2b) has been used to treat emerging and chronic viral diseases. However, rhIFN-α2b is immunogenic and has a short in vivo half-life. To solve these limitations, two long-lasting hyperglycosylated proteins with reduced immunogenicity were developed and designated as 4N-IFN(VAR1) and 4N-IFN(VAR3). Here, we continue to study the relevant characteristics of these therapeutic candidates. Thus, we demonstrated that both de-immunized IFN versions elicited significantly lower neutralizing antibody responses than the original molecule in HLA-DR1 transgenic mice, confirming our previous in vitro protein immunogenicity data. Also, we found that these biobetters exhibited remarkable stability when exposed to different physical factors that the protein product may encounter during its production process and storage, such as low pH, thermal stress, and repeated freezing/thawing cycles. Taking into consideration our previous and present results, 4N-IFN(VAR1) and 4N-IFN-4N(VAR3) appear to be valuable candidates for the treatment of human viral diseases.
Keywords: Anti-viral therapy; De-immunization; EpiMatrix; IFN alpha; Immunogenicity; In vivo assays.
©2023TheAuthors.PublishedbyElsevierLtd.
Conflict of interest statement
The authors declare the following conflict of interests: Author name; [Two of the contributing authors, Anne S. De Groot and William Martin are senior officers and shareholders at EpiVax, Inc., a privately owned biotechnology company located in Providence, RI. These authors acknowledge that there is a potential conflict of interest related to its relationship with EpiVax and attest that the work contained in this research report is free of any bias that might be associated with the commercial goals of the company.]
Figures




References
-
- Goldstein D., Laszlo J. Interferon therapy in cancer: from imaginon to interferon. Cancer Res. 1986;46:4315–4329. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

