Increased expression of glutathione peroxidase 3 prevents tendinopathy by suppressing oxidative stress
- PMID: 37021050
- PMCID: PMC10067742
- DOI: 10.3389/fphar.2023.1137952
Increased expression of glutathione peroxidase 3 prevents tendinopathy by suppressing oxidative stress
Abstract
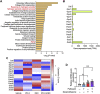
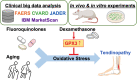
Tendinopathy, a degenerative disease, is characterized by pain, loss of tendon strength, or rupture. Previous studies have identified multiple risk factors for tendinopathy, including aging and fluoroquinolone use; however, its therapeutic target remains unclear. We analyzed self-reported adverse events and the US commercial claims data and found that the short-term use of dexamethasone prevented both fluoroquinolone-induced and age-related tendinopathy. Rat tendons treated systemically with fluoroquinolone exhibited mechanical fragility, histological change, and DNA damage; co-treatment with dexamethasone attenuated these effects and increased the expression of the antioxidant enzyme glutathione peroxidase 3 (GPX3), as revealed via RNA-sequencing. The primary role of GPX3 was validated in primary cultured rat tenocytes treated with fluoroquinolone or H2O2, which accelerates senescence, in combination with dexamethasone or viral overexpression of GPX3. These results suggest that dexamethasone prevents tendinopathy by suppressing oxidative stress through the upregulation of GPX3. This steroid-free approach for upregulation or activation of GPX3 can serve as a novel therapeutic strategy for tendinopathy.
Keywords: GPX3; aging; dexamethasone; fluoroquinolone; oxidative stress; real-world data; tendinopathy.
Copyright © 2023 Furuta, Yamada, Nagashima, Matsuda, Nagayasu, Shirakawa and Kaneko.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Alda-1, an activator of ALDH2, ameliorates Achilles tendinopathy in cellular and mouse models.Biochem Pharmacol. 2020 May;175:113919. doi: 10.1016/j.bcp.2020.113919. Epub 2020 Mar 17. Biochem Pharmacol. 2020. PMID: 32194057
-
Long noncoding RNA glutathione peroxidase 3-antisense inhibits lens epithelial cell apoptosis by upregulating glutathione peroxidase 3 expression in age-related cataract.Mol Vis. 2019 Nov 14;25:734-744. eCollection 2019. Mol Vis. 2019. PMID: 31814699 Free PMC article.
-
MiR-34a-5p Negatively Regulates Oxidative Stress on Lens Epithelial Cells by Silencing GPX3 - A Novel Target.Curr Eye Res. 2022 May;47(5):727-734. doi: 10.1080/02713683.2022.2029905. Epub 2022 Mar 22. Curr Eye Res. 2022. PMID: 35180020
-
Fluoroquinolone-Associated Tendinopathy: Does Levofloxacin Pose the Greatest Risk?Pharmacotherapy. 2016 Jun;36(6):679-93. doi: 10.1002/phar.1761. Epub 2016 Jun 11. Pharmacotherapy. 2016. PMID: 27138564 Review.
-
Fluoroquinolone-Mediated Tendinopathy and Tendon Rupture.Pharmaceuticals (Basel). 2025 Jan 30;18(2):184. doi: 10.3390/ph18020184. Pharmaceuticals (Basel). 2025. PMID: 40005998 Free PMC article. Review.
Cited by
-
Differential proteomic profiles between cognitive frail and robust older adults from the MELoR cohort.Geroscience. 2025 Jun;47(3):5069-5088. doi: 10.1007/s11357-024-01462-z. Epub 2024 Dec 9. Geroscience. 2025. PMID: 39653973 Free PMC article.
-
MicroRNA-146a gene transfer ameliorates senescence and senescence-associated secretory phenotypes in tendinopathic tenocytes.Aging (Albany NY). 2024 Feb 2;16(3):2702-2714. doi: 10.18632/aging.205505. Epub 2024 Feb 2. Aging (Albany NY). 2024. PMID: 38309291 Free PMC article.
-
Hypoxia-Inducible Factor and Oxidative Stress in Tendon Degeneration: A Molecular Perspective.Antioxidants (Basel). 2024 Jan 10;13(1):86. doi: 10.3390/antiox13010086. Antioxidants (Basel). 2024. PMID: 38247510 Free PMC article. Review.
-
Classification of distinct tendinopathy subtypes for precision therapeutics.Nat Commun. 2024 Nov 1;15(1):9460. doi: 10.1038/s41467-024-53826-w. Nat Commun. 2024. PMID: 39487125 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

