Effects of frog skin peptide temporin-1CEa and its analogs on ox-LDL induced macrophage-derived foam cells
- PMID: 37021059
- PMCID: PMC10067733
- DOI: 10.3389/fphar.2023.1139532
Effects of frog skin peptide temporin-1CEa and its analogs on ox-LDL induced macrophage-derived foam cells
Abstract
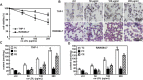
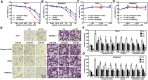
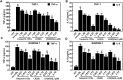
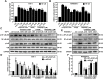
Purpose: Atherosclerosis is one of the most important pathological foundations of cardiovascular and cerebrovascular diseases with high morbidity and mortality. Studies have shown that macrophages play important roles in lipid accumulation in the vascular wall and thrombosis formation in atherosclerotic plaques. This study aimed to explore the effect of frog skin antimicrobial peptides (AMPs) temporin-1CEa and its analogs on ox-LDL induced macrophage-derived foam cells. Methods: CCK-8, ORO staining, and intracellular cholesterol measurements were used to study cellular activity, lipid droplet formation and cholesterol levels, respectively. ELISA, real-time quantitative PCR, Western blotting and flow cytometry analysis were used to study the expression of inflammatory factors, mRNA and proteins associated with ox-LDL uptake and cholesterol efflux in macrophage-derived foam cells, respectively. Furthermore, the effects of AMPs on inflammation signaling pathways were studied. Results: Frog skin AMPs could significantly increase the cell viability of the ox-LDL-induced foaming macrophages and decrease the formation of intracellular lipid droplets and the levels of total cholesterol and cholesterol ester (CE). Frog skin AMPs inhibited foaming formation by reducing the protein expression of CD36, which regulates ox-LDL uptake but had no effect on the expression of efflux proteins ATP binding cassette subfamily A/G member 1 (ABCA1/ABCG1). Then, decreased mRNA expression of NF-κB and protein expression of p-NF-κB p65, p-IκB, p-JNK, p-ERK, p-p38 and the release of TNF-α and IL-6 occurred after exposure to the three frog skin AMPs. Conclusion: Frog skin peptide temporin-1CEa and its analogs can improve the ox-LDL induced formation of macrophage-derived foam cells, in addition, inhibit inflammatory cytokine release through inhibiting the NF-κB and MAPK signaling pathways, thereby inhibiting inflammatory responses in atherosclerosis.
Keywords: atherosclerosis; foam cells; frog skin peptide; inflammation; lipid metabolism.
Copyright © 2023 Yang, Liu, Yan and Shang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
PRMT2 inhibits the formation of foam cell induced by ox-LDL in RAW 264.7 macrophage involving ABCA1 mediated cholesterol efflux.Biochem Biophys Res Commun. 2020 Mar 26;524(1):77-82. doi: 10.1016/j.bbrc.2020.01.040. Epub 2020 Jan 21. Biochem Biophys Res Commun. 2020. PMID: 31980179
-
Reduced beta2-glycoprotein I protects macrophages from ox-LDL-induced foam cell formation and cell apoptosis.Lipids Health Dis. 2013 Nov 16;12:174. doi: 10.1186/1476-511X-12-174. Lipids Health Dis. 2013. PMID: 24238298 Free PMC article.
-
Gly[14]-humanin inhibits ox-LDL uptake and stimulates cholesterol efflux in macrophage-derived foam cells.Biochem Biophys Res Commun. 2017 Jan 1;482(1):93-99. doi: 10.1016/j.bbrc.2016.10.138. Epub 2016 Nov 1. Biochem Biophys Res Commun. 2017. PMID: 27815075
-
Foam cells in atherosclerosis.Clin Chim Acta. 2013 Sep 23;424:245-52. doi: 10.1016/j.cca.2013.06.006. Epub 2013 Jun 16. Clin Chim Acta. 2013. PMID: 23782937 Review.
-
Attenuating lipid metabolism in atherosclerosis: The potential role of Anti-oxidative effects on low-density lipoprotein of herbal medicines.Front Pharmacol. 2023 Mar 31;14:1161657. doi: 10.3389/fphar.2023.1161657. eCollection 2023. Front Pharmacol. 2023. PMID: 37063287 Free PMC article. Review.
Cited by
-
Crosstalk between lipid metabolism and macrophages in atherosclerosis: therapeutic potential of natural products.Front Cardiovasc Med. 2025 Mar 3;12:1529924. doi: 10.3389/fcvm.2025.1529924. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40099271 Free PMC article. Review.
-
Chensinin-1b Alleviates DSS-Induced Inflammatory Bowel Disease by Inducing Macrophage Switching from the M1 to the M2 Phenotype.Biomedicines. 2024 Feb 1;12(2):345. doi: 10.3390/biomedicines12020345. Biomedicines. 2024. PMID: 38397947 Free PMC article.
-
The Contribution of Antimicrobial Peptides to Immune Cell Function: A Review of Recent Advances.Pharmaceutics. 2023 Sep 4;15(9):2278. doi: 10.3390/pharmaceutics15092278. Pharmaceutics. 2023. PMID: 37765247 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

