Somatostatin neuron contributions to cortical slow wave dysfunction in adult mice exposed to developmental ethanol
- PMID: 37021136
- PMCID: PMC10067632
- DOI: 10.3389/fnins.2023.1127711
Somatostatin neuron contributions to cortical slow wave dysfunction in adult mice exposed to developmental ethanol
Abstract
Introduction: Transitions between sleep and waking and sleep-dependent cortical oscillations are heavily dependent on GABAergic neurons. Importantly, GABAergic neurons are especially sensitive to developmental ethanol exposure, suggesting a potential unique vulnerability of sleep circuits to early ethanol. In fact, developmental ethanol exposure can produce long-lasting impairments in sleep, including increased sleep fragmentation and decreased delta wave amplitude. Here, we assessed the efficacy of optogenetic manipulations of somatostatin (SST) GABAergic neurons in the neocortex of adult mice exposed to saline or ethanol on P7, to modulate cortical slow-wave physiology.
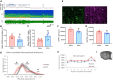
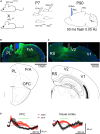
Methods: SST-cre × Ai32 mice, which selectively express channel rhodopsin in SST neurons, were exposed to ethanol or saline on P7. This line expressed similar developmental ethanol induced loss of SST cortical neurons and sleep impairments as C57BL/6By mice. As adults, optical fibers were implanted targeting the prefrontal cortex (PFC) and telemetry electrodes were implanted in the neocortex to monitor slow-wave activity and sleep-wake states.
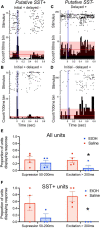
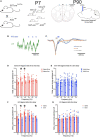
Results: Optical stimulation of PFC SST neurons evoked slow-wave potentials and long-latency single-unit excitation in saline treated mice but not in ethanol mice. Closed-loop optogenetic stimulation of PFC SST neuron activation on spontaneous slow-waves enhanced cortical delta oscillations, and this manipulation was more effective in saline mice than P7 ethanol mice.
Discussion: Together, these results suggest that SST cortical neurons may contribute to slow-wave impairment after developmental ethanol.
Keywords: GABA; closed loop optogenetics; cortical interneurons; fetal alcohol spectrum disorder (FASD); prefrontal cortex; slow wave sleep (SWS); somatostatin.
Copyright © 2023 Wilson, Fleming, Williams, Teixeira, Smiley and Saito.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

