Copeptin adaptive response to SGLT2 inhibitors in patients with type 2 diabetes mellitus: The GliRACo study
- PMID: 37021137
- PMCID: PMC10067557
- DOI: 10.3389/fnins.2023.1098404
Copeptin adaptive response to SGLT2 inhibitors in patients with type 2 diabetes mellitus: The GliRACo study
Abstract
Introduction: In type 2 diabetes mellitus (T2DM), the antidiuretic system participates in the adaptation to osmotic diuresis further increasing urinary osmolality by reducing the electrolyte-free water clearance. Sodium glucose co-transporter type 2 inhibitors (SGLT2i) emphasize this mechanism, promoting persistent glycosuria and natriuresis, but also induce a greater reduction of interstitial fluids than traditional diuretics. The preservation of osmotic homeostasis is the main task of the antidiuretic system and, in turn, intracellular dehydration the main drive to vasopressin (AVP) secretion. Copeptin is a stable fragment of the AVP precursor co-secreted with AVP in an equimolar amount.
Aim: To investigate the copeptin adaptive response to SGLT2i, as well as the induced changes in body fluid distribution in T2DM patients.
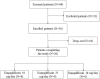
Methods: The GliRACo study was a prospective, multicenter, observational research. Twenty-six consecutive adult patients with T2DM were recruited and randomly assigned to empagliflozin or dapagliflozin treatment. Copeptin, plasma renin activity, aldosterone and natriuretic peptides were evaluated at baseline (T0) and then 30 (T30) and 90 days (T90) after SGLT2i starting. Bioelectrical impedance vector analysis (BIVA) and ambulatory blood pressure monitoring were performed at T0 and T90.
Results: Among endocrine biomarkers, only copeptin increased at T30, showing subsequent stability (7.5 pmol/L at T0, 9.8 pmol/L at T30, 9.5 pmol/L at T90; p = 0.001). BIVA recorded an overall tendency to dehydration at T90 with a stable proportion between extra- and intracellular fluid volumes. Twelve patients (46.1%) had a BIVA overhydration pattern at baseline and 7 of them (58.3%) resolved this condition at T90. Total body water content, extra and intracellular fluid changes were significantly affected by the underlying overhydration condition (p < 0.001), while copeptin did not.
Conclusion: In patients with T2DM, SGLT2i promote the release of AVP, thus compensating for persistent osmotic diuresis. This mainly occurs because of a proportional dehydration process between intra and extracellular fluid (i.e., intracellular dehydration rather than extracellular dehydration). The extent of fluid reduction, but not the copeptin response, is affected by the patient's baseline volume conditions.
Clinical trial registration: Clinicaltrials.gov, identifier NCT03917758.
Keywords: arginine-vasopressin; bioelectrical impedance vector analysis; extracellular fluid; osmotic homeostasis; renin-angiotensin-aldosterone system; sodium glucose co-transporter type 2 inhibitors.
Copyright © 2023 Berton, Parasiliti-Caprino, Prencipe, Bioletto, Lopez, Bona, Caputo, Rumbolo, Ponzetto, Settanni, Gasco, Mengozzi, Ghigo, Grottoli, Maccario and Benso.
Conflict of interest statement
AMB received fees from Thermo Fisher Diagnostics for previous editorial collaborations and oral presentations. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

