Spatial-dependent suppressive aftereffect produced by a sound in the rat's inferior colliculus is partially dependent on local inhibition
- PMID: 37021140
- PMCID: PMC10069703
- DOI: 10.3389/fnins.2023.1130892
Spatial-dependent suppressive aftereffect produced by a sound in the rat's inferior colliculus is partially dependent on local inhibition
Abstract
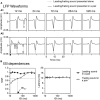
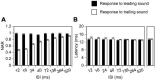
In a natural acoustic environment, a preceding sound can suppress the perception of a succeeding sound which can lead to auditory phenomena such as forward masking and the precedence effect. The degree of suppression is dependent on the relationship between the sounds in sound quality, timing, and location. Correlates of such phenomena exist in sound-elicited activities of neurons in hearing-related brain structures. The present study recorded responses to pairs of leading-trailing sounds from ensembles of neurons in the rat's inferior colliculus. Results indicated that a leading sound produced a suppressive aftereffect on the response to a trailing sound when the two sounds were colocalized at the ear contralateral to the site of recording (i.e., the ear that drives excitatory inputs to the inferior colliculus). The degree of suppression was reduced when the time gap between the two sounds was increased or when the leading sound was relocated to an azimuth at or close to the ipsilateral ear. Local blockage of the type-A γ-aminobutyric acid receptor partially reduced the suppressive aftereffect when a leading sound was at the contralateral ear but not at the ipsilateral ear. Local blockage of the glycine receptor partially reduced the suppressive aftereffect regardless of the location of the leading sound. Results suggest that a sound-elicited suppressive aftereffect in the inferior colliculus is partly dependent on local interaction between excitatory and inhibitory inputs which likely involves those from brainstem structures such as the superior paraolivary nucleus. These results are important for understanding neural mechanisms underlying hearing in a multiple-sound environment.
Keywords: GABAergic inhibition; binaural hearing; forward masking; free-field stimulation; glycinergic inhibition; inferior colliculus; sound location; suppressive aftereffect.
Copyright © 2023 Asim, Tran, Reynolds, Sauve and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








Similar articles
-
Glycine and GABA influence binaural processing in the inferior colliculus of the mustache bat.J Neurophysiol. 1995 Oct;74(4):1701-13. doi: 10.1152/jn.1995.74.4.1701. J Neurophysiol. 1995. PMID: 8989406
-
Spatial Separation between Two Sounds of an Oddball Paradigm Affects Responses of Neurons in the Rat's Inferior Colliculus to the Sounds.Neuroscience. 2020 Sep 15;444:118-135. doi: 10.1016/j.neuroscience.2020.07.027. Epub 2020 Jul 23. Neuroscience. 2020. PMID: 32712224
-
Two sources of inhibition affecting binaural evoked responses in the rat's inferior colliculus: the dorsal nucleus of the lateral lemniscus and the superior olivary complex.Hear Res. 1997 Feb;104(1-2):112-26. doi: 10.1016/s0378-5955(96)00182-7. Hear Res. 1997. PMID: 9119755
-
Auditory gating processes and binaural inhibition in the inferior colliculus.Hear Res. 2002 Jun;168(1-2):98-109. doi: 10.1016/s0378-5955(02)00356-8. Hear Res. 2002. PMID: 12117513 Review.
-
Circuits for processing dynamic interaural intensity disparities in the inferior colliculus.Hear Res. 2012 Jun;288(1-2):47-57. doi: 10.1016/j.heares.2012.01.011. Epub 2012 Feb 8. Hear Res. 2012. PMID: 22343068 Free PMC article. Review.
Cited by
-
Spatial separation between two sounds affects first-spike latencies of responses elicited by the sounds in the rat's auditory midbrain neurons.Sci Rep. 2025 Jul 8;15(1):24475. doi: 10.1038/s41598-025-03633-0. Sci Rep. 2025. PMID: 40628779 Free PMC article.
References
-
- Bregman A. S. (1990). Auditory scene analysis: The perceptual organization of sound. Cambridge, MA: MIT Press. 10.7551/mitpress/1486.001.0001 - DOI
-
- Bronkhorst A. W. (2000). The cocktail party phenomenon: A review of speech intelligibility in multiple-talker condition. Acta Acust. United Acust. 86 117–128.
LinkOut - more resources
Full Text Sources
Miscellaneous

