European Stroke Organisation (ESO) expedited recommendation on tenecteplase for acute ischaemic stroke
- PMID: 37021186
- PMCID: PMC10069183
- DOI: 10.1177/23969873221150022
European Stroke Organisation (ESO) expedited recommendation on tenecteplase for acute ischaemic stroke
Abstract
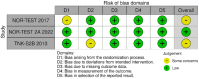
Within the last year, four randomised-controlled clinical trials (RCTs) have been published comparing intravenous thrombolysis (IVT) with tenecteplase and alteplase in acute ischaemic stroke (AIS) patients with a non-inferiority design for three of them. An expedited recommendation process was initiated by the European Stroke Organisation (ESO) and conducted according to ESO standard operating procedure based on the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework. We identified three relevant Population, Intervention, Comparator, Outcome (PICO) questions, performed systematic reviews of the literature and meta-analyses, assessed the quality of the available evidence, and wrote evidence-based recommendations. Expert consensus statements were provided if insufficient evidence was available to provide recommendations based on the GRADE approach. For patients with AIS of <4.5 h duration who are eligible for IVT, tenecteplase 0.25 mg/kg can be used as a safe and effective alternative to alteplase 0.9 mg/kg (moderate evidence, strong recommendation). For patients with AIS of <4.5 h duration who are eligible for IVT, we recommend against using tenecteplase at a dose of 0.40 mg/kg (low evidence, strong recommendation). For patients with AIS of <4.5 h duration with prehospital management with a mobile stroke unit who are eligible for IVT, we suggest tenecteplase 0.25 mg/kg over alteplase 0.90 mg/kg (low evidence, weak recommendation). For patients with large vessel occlusion (LVO) AIS of <4.5 h duration who are eligible for IVT, we recommend tenecteplase 0.25 mg/kg over alteplase 0.9 mg/kg (moderate evidence, strong recommendation). For patients with AIS on awakening from sleep or AIS of unknown onset who are selected with non-contrast CT, we recommend against IVT with tenecteplase 0.25 mg/kg (low evidence, strong recommendation). Expert consensus statements are also provided. Tenecteplase 0.25 mg/kg may be favoured over alteplase 0.9 mg/kg for patients with AIS of <4.5 h duration in view of comparable safety and efficacy data and easier administration. For patients with LVO AIS of <4.5 h duration who are IVT-eligible, IVT with tenecteplase 0.25 mg/kg is preferable over skipping IVT before MT, even in the setting of a direct admission to a thrombectomy-capable centre. IVT with tenecteplase 0.25 mg/kg may be a reasonable alternative to alteplase 0.9 mg/kg for patients with AIS on awakening from sleep or AIS of unknown onset and who are IVT-eligible after selection with advanced imaging.
Keywords: European Stroke Organisation; Intravenous thrombolysis; acute ischaemic stroke; extended time window; large vessel occlusion; recommendations; tenecteplase; wake-up stroke.
© European Stroke Organisation 2023.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Intellectual and financial disclosures of the module working group members are presented in Supplemental Table.
Figures















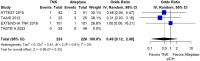


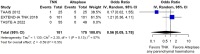





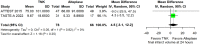



































References
-
- Thiebaut AM, Gauberti M, Ali C, et al. . The role of plasminogen activators in stroke treatment: fibrinolysis and beyond. Lancet Neurol 2018; 17: 1121–1132. - PubMed
-
- Assessment of the Safety and Efficacy of a New Thrombolytic (ASSENT-2) Investigators, Van de Werf F. Single-bolus tenecteplase compared with front-loaded alteplase in acute myocardial infarction: the ASSENT-2 double-blind randomised trial. Lancet 1999; 354: 716–722. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

