Maternal SARS-CoV-2, Placental Changes and Brain Injury in 2 Neonates
- PMID: 37021494
- PMCID: PMC10467358
- DOI: 10.1542/peds.2022-058271
Maternal SARS-CoV-2, Placental Changes and Brain Injury in 2 Neonates
Abstract
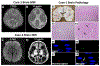
Long-term neurodevelopmental sequelae are a potential concern in neonates following in utero exposure to severe acute respiratory syndrome coronavirus disease 2 (SARS-CoV-2). We report 2 neonates born to SARS-CoV-2 positive mothers, who displayed early-onset (day 1) seizures, acquired microcephaly, and significant developmental delay over time. Sequential MRI showed severe parenchymal atrophy and cystic encephalomalacia. At birth, neither infant was SARS-CoV-2 positive (nasopharyngeal swab, reverse transcription polymerase chain reaction), but both had detectable SARS-CoV-2 antibodies and increased blood inflammatory markers. Placentas from both mothers showed SARS-CoV-2-nucleocapsid protein and spike glycoprotein 1 in the syncytiotrophoblast, fetal vascular malperfusion, and significantly increased inflammatory and oxidative stress markers pyrin domain containing 1 protein, macrophage inflammatory protein 1 βη, stromal cell-derived factor 1, interleukin 13, and interleukin 10, whereas human chorionic gonadotropin was markedly decreased. One infant (case 1) experienced sudden unexpected infant death at 13 months of age. The deceased infant's brain showed evidence of SARS-CoV-2 by immunofluorescence, with colocalization of the nucleocapsid protein and spike glycoprotein around the nucleus as well as within the cytoplasm. The constellation of clinical findings, placental pathology, and immunohistochemical changes strongly suggests that second-trimester maternal SARS-CoV-2 infection with placentitis triggered an inflammatory response and oxidative stress injury to the fetoplacental unit that affected the fetal brain. The demonstration of SARS-CoV-2 in the deceased infant's brain also raises the possibility that SARS-CoV-2 infection of the fetal brain directly contributed to ongoing brain injury. In both infants, the neurologic findings at birth mimicked the presentation of hypoxic-ischemic encephalopathy of newborn and neurologic sequelae progressed well beyond the neonatal period.
Conflict of interest statement
Dr Paidas has received funding from BioIncept, LLC to study hypoxic-ischemic encephalopathy with Preimplantation Factor. Dr Paidas is Scientific Advisory Board Member, BioIncept, LLC and has stock options. Drs Paidas and Jayakumar are co-inventors of SPIKENET, University of Miami, patent pending 2023. The other authors have no conflicts of interest to disclose.
Figures

References
-
- Jayakumar AR, Tong XY Shamaladevi N, et al. Defective synthesis and release of astrocytic thrombospondin-1 mediates the neuronal TDP-43 proteinopathy, resulting in defects in neuronal integrity associated with chronic traumatic encephalopathy: in vitro studies. J Neurochem. 2017;140(4):645–661 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

