Establishment and Utility of SwedAD: A Nationwide Swedish Registry for Patients with Atopic Dermatitis Receiving Systemic Pharmacotherapy
- PMID: 37021597
- PMCID: PMC10108712
- DOI: 10.2340/actadv.v103.7312
Establishment and Utility of SwedAD: A Nationwide Swedish Registry for Patients with Atopic Dermatitis Receiving Systemic Pharmacotherapy
Abstract
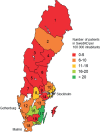
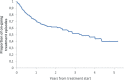
SwedAD, a Swedish nationwide registry for patients with atopic dermatitis receiving systemic pharmacotherapy, was launched on 1 September 2019. We describe here the establishment of a user-friendly registry to the benefit of patients with atopic dermatitis. By 5 November 2022, 38 clinics had recorded 931 treatment episodes in 850 patients with an approximate national coverage rate of 40%. Characteristics at enrolment included median Eczema Area and Severity Index (EASI) 10.2 (interquartile range 4.0, 19.4), Patient-Oriented Eczema Measure (POEM) 18.0 (10.0, 24.0), Dermatology Life Quality Index (DLQI) 11.0 (5.0, 19.0) and Peak Itch Numerical Rating Scale-11 (NRS-11) 6.0 (3.0, 8.0). At 3 months, median EASI was 3.2 (1.0, 7.3) and POEM, DLQI, and NRS-11 were improved. Regional coverage varied, reflecting the distribution of dermatologists, the ratio of public to private healthcare, and difficulties in recruiting certain clinics. This study highlights the importance of a nationwide registry when managing systemic pharmacotherapy of atopic dermatitis.
Conflict of interest statement
MA has received speaker honoraria and/or been in advisory boards for AbbVie, Eli-Lilly, LEO Pharma, Pfizer, and Sanofi-Genzyme, and is/has been an investigator for AbbVie, and Sanofi-Genzyme. AS has received speaker honoraria from Janssen and been a consultant for AbbVie, Eli Lilly, ICON plc, and Novartis. LHS has received speaker honoraria and/or been in advisory boards for Abbvie, Eli-Lilly, LeoPharma, Pfizer, and Sanofi-Genzyme. LUI has received speaker honoraria and/or been a consultant for AbbVie, ACO, LEO Pharma, Novartis, and Sanofi-Genzyme. AJ has received speaker honoraria and/or been in advisory boards for AbbVie, ACO, Eli-Lilly, and Sanofi-Genzyme. LvK has been a speaker, advisory board member, and/or investigator for Eli Lilly, Leo Pharma, Pfizer, and Sanofi-Genzyme. ML has received speaker honoraria from AbbVie and Sanofi-Genzyme. MHSF has received speaker honoraria and/or been in advisory boards for Almirall, Astellas, AstraZeneca, AbbVie, Celgene, Galderma, LEO Pharma, Lilly, MEDA, Novartis, Sanofi-Genzyme, and Wyeth. GS has received speaker honoraria from AbbVie. ASn has received speaker honoraria and consulting fees from AbbVie, LEO Pharma, Pfizer, and Sanofi. Payments were made to ASn’s institution. ASn has been an investigator for AbbVie. SV has received speaker honoraria and been a consultant for Sanofi Genzyme and has received speaker honoraria which were paid to Atopikerna from LEO Pharma and Sanofi-Genzyme. C-FW has participated in expert meetings with AbbVie and Sanofi-Genzyme without receiving personal financial compensation. MB has received speaker honoraria and/or been in advisory boards for AbbVie, ACO, LEO Pharma, Pfizer, Novartis, and Sanofi-Genzyme, and is an investigator for AbbVie. EKJ has received speaker honoraria and/or been a consultant for AbbVie, ACO, Galenica, LEO Pharma, Novartis, and Sanofi-Genzyme.
Figures


References
-
- Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers 2018; 4: 1. - PubMed
-
- Weidinger S, Novak N. Atopic dermatitis. Lancet 2016; 387: 1109–1122. - PubMed
-
- Flohr C, Irvine AD. Systemic therapies for severe atopic dermatitis in children and adults. J Allergy Clin Immunol 2013; 132: 774–774.e6. - PubMed
-
- McAleer MA, Flohr C, Irvine AD. Management of difficult and severe eczema in childhood. BMJ 2012; 345: e4770. - PubMed
-
- Schmitt J, Spuls PI, Thomas KS, Simpson E, Furue M, Deckert S, et al. . The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol 2014; 134: 800–807. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

