Expression and characterisation of human glycerol kinase: the role of solubilising agents and molecular chaperones
- PMID: 37021775
- PMCID: PMC10130975
- DOI: 10.1042/BSR20222258
Expression and characterisation of human glycerol kinase: the role of solubilising agents and molecular chaperones
Erratum in
-
Correction: Expression and characterisation of human glycerol kinase: the role of solubilising agents and molecular chaperones.Biosci Rep. 2023 Oct 31;43(10):BSR-2022-2258_COR. doi: 10.1042/BSR-2022-2258_COR. Biosci Rep. 2023. PMID: 37860917 Free PMC article. No abstract available.
Abstract
Background: Glycerol kinase (GK; EC 2.7.1.30) facilitates the entry of glycerol into pathways of glucose and triglyceride metabolism and may play a potential role in Type 2 diabetes mellitus (T2DM). However, the detailed regulatory mechanisms and structure of the human GK are unknown.
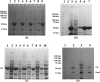
Methods: The human GK gene was cloned into the pET-24a(+) vector and over-expressed in Escherichia coli BL21 (DE3). Since the protein was expressed as inclusion bodies (IBs), various culture parameters and solubilising agents were used but they did not produce bioactive His-GK; however, co-expression of His-GK with molecular chaperones, specifically pKJE7, achieved expression of bioactive His-GK. The overexpressed bioactive His-GK was purified using coloumn chromatography and characterised using enzyme kinetics.
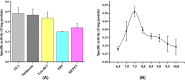
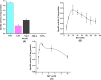
Results: The overexpressed bioactive His-GK was purified apparently to homogeneity (∼295-fold) and characterised. The native His-GK was a dimer with a monomeric molecular weight of ∼55 kDa. Optimal enzyme activity was observed in TEA buffer (50 mM) at 7.5 pH. K+ (40 mM) and Mg2+ (2.0 mM) emerged as prefered metal ions for His-GK activity with specific activity 0.780 U/mg protein. The purified His-GK obeyed standard Michaelis-Menten kinetics with Km value of 5.022 µM (R2=0.927) for its substrate glycerol; whereas, that for ATP and PEP was 0.767 mM (R2=0.928) and 0.223 mM (R2=0.967), respectively. Other optimal parameters for the substrate and co-factors were also determined.
Conclusion: The present study demonstrates that co-expression of molecular chaperones assists with the expression of bioactive human GK for its characterisation.
Keywords: Type 2 diabetes mellitus; glycerol kinase; molecular chaperones; sarkosyl; solubilising agents.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures







Similar articles
-
Thermostable glycerol kinase from a hyperthermophilic archaeon: gene cloning and characterization of the recombinant enzyme.Protein Eng. 1998 Dec;11(12):1219-27. doi: 10.1093/protein/11.12.1219. Protein Eng. 1998. PMID: 9930671
-
Crystal structure of highly thermostable glycerol kinase from a hyperthermophilic archaeon in a dimeric form.FEBS J. 2008 May;275(10):2632-43. doi: 10.1111/j.1742-4658.2008.06410.x. Epub 2008 Apr 17. FEBS J. 2008. PMID: 18422647
-
The ATP-stimulated translocation promoter (ASTP) activity of glycerol kinase plays central role in adipogenesis.Mol Genet Metab. 2018 Aug;124(4):254-265. doi: 10.1016/j.ymgme.2018.06.001. Epub 2018 Jun 12. Mol Genet Metab. 2018. PMID: 29960856
-
Affinity shift of ATP upon glycerol binding to a glycerol kinase from the hyperthermophilic archaeon Thermococcus kodakarensis KOD1.J Biosci Bioeng. 2020 Jun;129(6):657-663. doi: 10.1016/j.jbiosc.2019.12.008. Epub 2020 Jan 31. J Biosci Bioeng. 2020. PMID: 32008925
-
Recent Developments in Medicinal Chemistry of Allosteric Activators of Human Glucokinase for Type 2 Diabetes Mellitus Therapeutics.Curr Pharm Des. 2020;26(21):2510-2552. doi: 10.2174/1381612826666200414163148. Curr Pharm Des. 2020. PMID: 32286938 Review.
Cited by
-
Glycylglycine promotes the solubility and antigenic utility of recombinant HCV structural proteins in a point-of-care immunoassay for detection of active viremia.Microb Cell Fact. 2024 Jan 18;23(1):25. doi: 10.1186/s12934-024-02297-1. Microb Cell Fact. 2024. PMID: 38238770 Free PMC article.
-
TRIP6 a potential diagnostic marker for colorectal cancer with glycolysis and immune infiltration association.Sci Rep. 2024 Feb 19;14(1):4042. doi: 10.1038/s41598-024-54670-0. Sci Rep. 2024. PMID: 38369589 Free PMC article.
-
Sodium Dodecyl Sulfate Analogs as a Potential Molecular Biology Reagent.Curr Issues Mol Biol. 2024 Jan 9;46(1):621-633. doi: 10.3390/cimb46010040. Curr Issues Mol Biol. 2024. PMID: 38248342 Free PMC article. Review.
-
Functional and Biochemical Analyses of Glycerol Kinase and Glycerol 3-phosphate Dehydrogenase in HEK293 Cells.Protein J. 2025 Apr;44(2):231-244. doi: 10.1007/s10930-025-10252-1. Epub 2025 Feb 22. Protein J. 2025. PMID: 39987391
-
Biochemical properties of glycerol kinase from the hypersaline-adapted archaeon Haloferax volcanii.Appl Environ Microbiol. 2025 Aug 20;91(8):e0088625. doi: 10.1128/aem.00886-25. Epub 2025 Jul 8. Appl Environ Microbiol. 2025. PMID: 40626661 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

