Role of HNF4alpha-cMyc interaction in liver regeneration and recovery after acetaminophen-induced acute liver injury
- PMID: 37021787
- PMCID: PMC10523339
- DOI: 10.1097/HEP.0000000000000367
Role of HNF4alpha-cMyc interaction in liver regeneration and recovery after acetaminophen-induced acute liver injury
Abstract
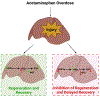
Background and aims: Overdose of acetaminophen (APAP) is the major cause of acute liver failure in the western world. We report a novel signaling interaction between hepatocyte nuclear factor 4 alpha (HNF4α) cMyc and nuclear factor erythroid 2-related factor 2 (Nrf2) during liver injury and regeneration after APAP overdose.
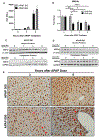
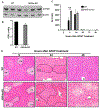
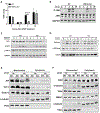
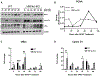
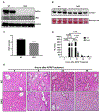
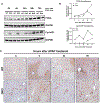
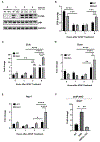
Approach and results: APAP-induced liver injury and regeneration were studied in male C57BL/6J (WT) mice, hepatocyte-specific HNF4α knockout mice (HNF4α-KO), and HNF4α-cMyc double knockout mice (DKO). C57BL/6J mice treated with 300 mg/kg maintained nuclear HNF4α expression and exhibited liver regeneration, resulting in recovery. However, treatment with 600-mg/kg APAP, where liver regeneration was inhibited and recovery was delayed, showed a rapid decline in HNF4α expression. HNF4α-KO mice developed significantly higher liver injury due to delayed glutathione recovery after APAP overdose. HNF4α-KO mice also exhibited significant induction of cMyc, and the deletion of cMyc in HNF4α-KO mice (DKO mice) reduced the APAP-induced liver injury. The DKO mice had significantly faster glutathione replenishment due to rapid induction in Gclc and Gclm genes. Coimmunoprecipitation and ChIP analyses revealed that HNF4α interacts with Nrf2 and affects its DNA binding. Furthermore, DKO mice showed significantly faster initiation of cell proliferation resulting in rapid liver regeneration and recovery.
Conclusions: These data show that HNF4α interacts with Nrf2 and promotes glutathione replenishment aiding in recovery from APAP-induced liver injury, a process inhibited by cMyc. These studies indicate that maintaining the HNF4α function is critical for regeneration and recovery after APAP overdose.
Copyright © 2023 American Association for the Study of Liver Diseases.
Conflict of interest statement
Conflict of interest:
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Comment on
-
Head-to-head comparison of magnetic resonance elastography-based liver stiffness, fat fraction, and T1 relaxation time in identifying at-risk NASH.Hepatology. 2023 Oct 1;78(4):1200-1208. doi: 10.1097/HEP.0000000000000417. Epub 2023 May 1. Hepatology. 2023. PMID: 37080558 Free PMC article. Clinical Trial.
References
-
- Bernal W and Wendon J, Acute liver failure. N Engl J Med, 2013. 369(26): p. 2525–34. - PubMed
-
- Larson AM, et al., Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology, 2005. 42(6): p. 1364–72. - PubMed
-
- Jaeschke H and Bajt ML, Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci, 2006. 89(1): p. 31–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

