Impact of Intestinal Microbiota on Growth Performance of Suckling and Weaned Piglets
- PMID: 37022154
- PMCID: PMC10269657
- DOI: 10.1128/spectrum.03744-22
Impact of Intestinal Microbiota on Growth Performance of Suckling and Weaned Piglets
Abstract
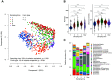
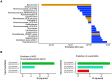
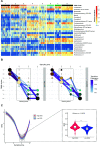
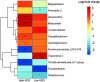
Small-scale studies investigating the relationship between pigs' intestinal microbiota and growth performance have generated inconsistent results. We hypothesized that on farms under favorable environmental conditions (e.g., promoting sow nest-building behavior, high colostrum production, low incidence of diseases and minimal use of antimicrobials), the piglet gut microbiota may develop toward a population that promotes growth and reduces pathogenic bacteria. Using 16S rRNA gene amplicon sequencing, we sampled and profiled the fecal microbiota from 170 individual piglets throughout suckling and postweaning periods (in total 670 samples) to track gut microbiota development and its potential association with growth. During the suckling period, the dominant genera were Lactobacillus and Bacteroides, the latter being gradually replaced by Clostridium sensu scricto 1 as piglets aged. The gut microbiota during the nursery stage, not the suckling period, predicted the average daily growth (ADG) of piglets. The relative abundances of SCFA-producing genera, in particular Faecalibacterium, Megasphaera, Mitsuokella, and Subdoligranulum, significantly correlated with high ADG of weaned piglets. In addition, the succession of the gut microbiota in high-ADG piglets occurred faster and stabilized sooner upon weaning, whereas the gut microbiota of low-ADG piglets continued to mature after weaning. Overall, our findings suggest that weaning is the major driver of gut microbiota variation in piglets with different levels of overall growth performance. This calls for further research to verify if promotion of specific gut microbiota, identified here at weaning transition, is beneficial for piglet growth. IMPORTANCE The relationship between pigs' intestinal microbiota and growth performance is of great importance for improving piglets' health and reducing antimicrobial use. We found that gut microbiota variation is significantly associated with growth during weaning and the early nursery period. Importantly, transitions toward a mature gut microbiota enriched with fiber-degrading bacteria mostly complete upon weaning in piglets with better growth. Postponing the weaning age may therefore favor the development of fiber degrading gut bacteria, conferring the necessary capacity to digest and harvest solid postweaning feed. The bacterial taxa associated with piglet growth identified herein hold potential to improve piglet growth and health.
Keywords: NGS sequencing; SCFA; fiber degrading bacteria; growth; gut microbiota; pig; weaning.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Zhang Y, Liu Q, Zhang W, Zhang Z, Wang W, Zhuang S. 2018. Gastrointestinal microbial diversity and short-chain fatty acid production in pigs fed different fibrous diets with or without cell wall-degrading enzyme supplementation. Livestock Science 207:105–116. doi: 10.1016/j.livsci.2017.11.017. - DOI
-
- Bian G, Ma S, Zhu Z, Su Y, Zoetendal EG, Mackie R, Liu J, Mu C, Huang R, Smidt H, Zhu W. 2016. Age, introduction of solid feed and weaning are more important determinants of gut bacterial succession in piglets than breed and nursing mother as revealed by a reciprocal cross-fostering model. Environ Microbiol 18:1566–1577. doi: 10.1111/1462-2920.13272. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

