Mutant Prevention Concentration, Frequency of Spontaneous Mutant Selection, and Mutant Selection Window-a New Approach to the In Vitro Determination of the Antimicrobial Potency of Compounds
- PMID: 37022162
- PMCID: PMC10190592
- DOI: 10.1128/aac.01373-22
Mutant Prevention Concentration, Frequency of Spontaneous Mutant Selection, and Mutant Selection Window-a New Approach to the In Vitro Determination of the Antimicrobial Potency of Compounds
Abstract
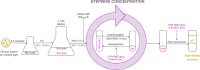
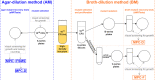
The analysis of antimicrobial activity is usually MIC- and minimal bactericidal concentration (MBC)-focused, though also crucial are resistance-related parameters, e.g., the frequency of spontaneous mutant selection (FSMS), the mutant prevention concentration (MPC), and the mutant selection window (MSW). In vitro-determined MPCs, however, are sometimes variable, poorly repeatable, and not always reproducible in vivo. We propose a new approach to the in vitro determination of MSWs, along with novel parameters: MPC-D, MSW-D (for dominant mutants, i.e., selected with a high frequency, without a fitness loss), and MPC-F, MSW-F (for inferior mutants, i.e., with an impaired fitness). We also propose a new method for preparing the high-density inoculum (>1011 CFU/mL). In this study, the MPC and MPC-D (limited by FSMS of <10-10) of ciprofloxacin, linezolid, and novel benzosiloxaborole (No37) were determined for Staphylococcus aureus ATCC 29213 using the standard agar method, while the MPC-D and MPC-F were determined by the novel broth method. Regardless of the method, MSWs1010 of linezolid and No37 were the same. However, MSWs1010 of ciprofloxacin in the broth method was narrower than in the agar method. In the broth method, the 24-h incubation of ~1010 CFU in a drug-containing broth differentiates the mutants that can dominate the cell population from those that can only be selected under exposure. We consider MPC-Ds in the agar method to be less variable and more repeatable than MPCs. Meanwhile, the broth method may decrease discrepancies between in vitro and in vivo MSWs. The proposed approaches may help establish MPC-D-related resistance-restricting therapies.
Keywords: FSMS; MPC; MPC-D; MPC-F; MSW; MSW-D; MSW-F; antibiotic resistance; antimicrobial activity; antimicrobial agents.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
In Vitro Resistance-Predicting Studies and In Vitro Resistance-Related Parameters-A Hit-to-Lead Perspective.Pharmaceuticals (Basel). 2024 Aug 15;17(8):1068. doi: 10.3390/ph17081068. Pharmaceuticals (Basel). 2024. PMID: 39204172 Free PMC article. Review.
-
Enrichment of fluoroquinolone-resistant Staphylococcus aureus: oscillating ciprofloxacin concentrations simulated at the upper and lower portions of the mutant selection window.Antimicrob Agents Chemother. 2008 Jun;52(6):1924-8. doi: 10.1128/AAC.01371-07. Epub 2008 Mar 31. Antimicrob Agents Chemother. 2008. PMID: 18378704 Free PMC article.
-
Testing the mutant selection window hypothesis with Staphylococcus aureus exposed to linezolid in an in vitro dynamic model.J Antimicrob Chemother. 2017 Nov 1;72(11):3100-3107. doi: 10.1093/jac/dkx249. J Antimicrob Chemother. 2017. PMID: 28981793
-
[Mutant prevention concentrations of fluoroquinolones for Staphylococcus aureus].Zhonghua Yi Xue Za Zhi. 2004 Nov 17;84(22):1863-6. Zhonghua Yi Xue Za Zhi. 2004. PMID: 15631794 Chinese.
-
The role of PK/PD parameters to avoid selection and increase of resistance: mutant prevention concentration.J Chemother. 2004 Jun;16 Suppl 3:1-19. doi: 10.1080/1120009x.2004.11782371. J Chemother. 2004. PMID: 15334827 Review.
Cited by
-
In Vitro Resistance-Predicting Studies and In Vitro Resistance-Related Parameters-A Hit-to-Lead Perspective.Pharmaceuticals (Basel). 2024 Aug 15;17(8):1068. doi: 10.3390/ph17081068. Pharmaceuticals (Basel). 2024. PMID: 39204172 Free PMC article. Review.
-
Ethynyl-substituted benzosiloxaboroles: the role of C(π)⋯B interactions in their crystal packing and use in Cu(i)-catalyzed 1,3-dipolar cycloaddition.RSC Adv. 2024 May 17;14(23):16069-16082. doi: 10.1039/d4ra02137a. eCollection 2024 May 15. RSC Adv. 2024. PMID: 38765480 Free PMC article.
-
Alternate Antimicrobial Therapies and Their Companion Tests.Diagnostics (Basel). 2023 Jul 26;13(15):2490. doi: 10.3390/diagnostics13152490. Diagnostics (Basel). 2023. PMID: 37568853 Free PMC article. Review.
-
Impact of Environmental Sub-Inhibitory Concentrations of Antibiotics, Heavy Metals, and Biocides on the Emergence of Tolerance and Effects on the Mutant Selection Window in E. coli.Microorganisms. 2023 Sep 9;11(9):2265. doi: 10.3390/microorganisms11092265. Microorganisms. 2023. PMID: 37764108 Free PMC article.
-
Synergistic antibacterial activity and prevention of drug resistance of daptomycin combined with fosfomycin against methicillin-resistant Staphylococcus aureus.Antimicrob Agents Chemother. 2025 Aug 6;69(8):e0160924. doi: 10.1128/aac.01609-24. Epub 2025 Jun 18. Antimicrob Agents Chemother. 2025. PMID: 40530996 Free PMC article.
References
-
- WHO. 2022. 2021 Antibacterial agents in clinical and preclinical development: an overview and analysis. World Health Organization, Geneva, Switzerland. https://www.who.int/publications/i/item/9789240047655.
-
- FDA. 2022. Antibacterial therapies for patients with an unmet medical need for the treatment of serious bacterial diseases—questions and answers (revision 1): guidance for industry. FDA-2013-D-0744. U.S. Food and Drug Administration, Silver Spring, MD.
-
- FDA. 2017. Antibacterial therapies for patients with an unmet medical need for the treatment of serious bacterial diseases. FDA-2013-D-0744. US Food and Drug Administration, Silver Spring, MD.
-
- Krajewska J, Laudy AE. 2021. The European Medicines Agency approved the new antibacterial drugs—response to the 2017 WHO report on the global problem of multi-drug resistance. Advancements Microbiol 60:249–264. doi:10.21307/pm-2021.60.4.20. - DOI
-
- CLSI. 1999. Methods for determining bactericidal activity of antimicrobial agents. Approved guideline. CLSI guideline M26-A. Clinical and Laboratory Standards Institute, Wayne, PA.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

