HtpG Is a Metal-Dependent Chaperone Which Assists the DnaK/DnaJ/GrpE Chaperone System of Mycobacterium tuberculosis via Direct Association with DnaJ2
- PMID: 37022172
- PMCID: PMC10269695
- DOI: 10.1128/spectrum.00312-23
HtpG Is a Metal-Dependent Chaperone Which Assists the DnaK/DnaJ/GrpE Chaperone System of Mycobacterium tuberculosis via Direct Association with DnaJ2
Abstract
Protein folding is a crucial process in maintaining protein homeostasis, also known as proteostasis, in the cell. The requirement for the assistance of molecular chaperones in the appropriate folding of several proteins has already called into question the previously held view of spontaneous protein folding. These chaperones are highly ubiquitous cellular proteins, which not only help in mediating the proper folding of other nascent polypeptides but are also involved in refolding of the misfolded or the aggregated proteins. Hsp90 family proteins such as high-temperature protein G (HtpG) are abundant and ubiquitously expressed in both eukaryotic and prokaryotic cells. Although HtpG is known as an ATP-dependent chaperone protein in most organisms, function of this protein remains obscured in mycobacterial pathogens. Here, we aim to investigate significance of HtpG as a chaperone in the physiology of Mycobacterium tuberculosis. We report that M. tuberculosis HtpG (mHtpG) is a metal-dependent ATPase which exhibits chaperonin activity towards denatured proteins in coordination with the DnaK/DnaJ/GrpE chaperone system via direct association with DnaJ2. Increased expression of DnaJ1, DnaJ2, ClpX, and ClpC1 in a ΔhtpG mutant strain further suggests cooperativity of mHtpG with various chaperones and proteostasis machinery in M. tuberculosis. IMPORTANCE M. tuberculosis is exposed to variety of extracellular stressful conditions and has evolved mechanisms to endure and adapt to the adverse conditions for survival. mHtpG, despite being dispensable for M. tuberculosis growth under in vitro conditions, exhibits a strong and direct association with DnaJ2 cochaperone and assists the mycobacterial DnaK/DnaJ/GrpE (KJE) chaperone system. These findings suggest the potential role of mHtpG in stress management of the pathogen. Mycobacterial chaperones are responsible for folding of nascent protein as well as reactivation of protein aggregates. M. tuberculosis shows differential adaptive response subject to the availability of mHtpG. While its presence facilitates improved protein refolding via stimulation of the KJE chaperone activity, in the absence of mHtpG, M. tuberculosis enhances expression of DnaJ1/J2 cochaperones as well as Clp protease machinery for maintenance of proteostasis. Overall, this study provides a framework for future investigation to better decipher the mycobacterial proteostasis network in the light of stress adaptability and/or survival.
Keywords: Clp protease; DnaJ2; DnaK; HSP90; HtpG; Mycobacterium tuberculosis; chaperones; heat shock proteins; proteostasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

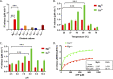
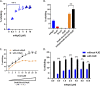




Similar articles
-
Reconstitution of a Mycobacterium tuberculosis proteostasis network highlights essential cofactor interactions with chaperone DnaK.Proc Natl Acad Sci U S A. 2016 Dec 6;113(49):E7947-E7956. doi: 10.1073/pnas.1617644113. Epub 2016 Nov 21. Proc Natl Acad Sci U S A. 2016. PMID: 27872278 Free PMC article.
-
ClpB and HtpG facilitate de novo protein folding in stressed Escherichia coli cells.Mol Microbiol. 2000 Jun;36(6):1360-70. doi: 10.1046/j.1365-2958.2000.01951.x. Mol Microbiol. 2000. PMID: 10931286
-
Temperature-controlled activity of DnaK-DnaJ-GrpE chaperones: protein-folding arrest and recovery during and after heat shock depends on the substrate protein and the GrpE concentration.Biochemistry. 1998 Jul 7;37(27):9688-94. doi: 10.1021/bi980338u. Biochemistry. 1998. PMID: 9657681
-
Chaperone-assisted protein folding in the cell cytoplasm.Curr Protein Pept Sci. 2001 Sep;2(3):227-44. doi: 10.2174/1389203013381134. Curr Protein Pept Sci. 2001. PMID: 12369934 Review.
-
The Bacterial Hsp90 Chaperone: Cellular Functions and Mechanism of Action.Annu Rev Microbiol. 2021 Oct 8;75:719-739. doi: 10.1146/annurev-micro-032421-035644. Epub 2021 Aug 10. Annu Rev Microbiol. 2021. PMID: 34375543 Review.
Cited by
-
Analysis of the components of Mycobacterium tuberculosis heat-resistant antigen (Mtb-HAg) and its regulation of γδ T-cell function.Cell Mol Biol Lett. 2024 May 13;29(1):70. doi: 10.1186/s11658-024-00585-7. Cell Mol Biol Lett. 2024. PMID: 38741147 Free PMC article.
-
Hsp90, DnaK, and ClpB collaborate in protein reactivation.Proc Natl Acad Sci U S A. 2025 Feb 4;122(5):e2422640122. doi: 10.1073/pnas.2422640122. Epub 2025 Jan 29. Proc Natl Acad Sci U S A. 2025. PMID: 39879241 Free PMC article.
-
Surfing in the storm: how Paraburkholderia xenovorans thrives under stress during biodegradation of toxic aromatic compounds and other stressors.FEMS Microbiol Rev. 2025 Jan 14;49:fuaf021. doi: 10.1093/femsre/fuaf021. FEMS Microbiol Rev. 2025. PMID: 40388301 Free PMC article. Review.
-
HtpG-A Major Virulence Factor and a Promising Vaccine Antigen against Mycobacterium tuberculosis.Biomolecules. 2024 Apr 11;14(4):471. doi: 10.3390/biom14040471. Biomolecules. 2024. PMID: 38672487 Free PMC article. Review.
-
Hsp90, a team player in protein quality control and the stress response in bacteria.Microbiol Mol Biol Rev. 2024 Jun 27;88(2):e0017622. doi: 10.1128/mmbr.00176-22. Epub 2024 Mar 27. Microbiol Mol Biol Rev. 2024. PMID: 38534118 Free PMC article. Review.
References
-
- Nover L, Hellmund D, Neumann D, Scharf K, Serfling E. 1984. The heat shock response of eukaryotic cells. Biologisches Zentralblatt 103:357–435.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

