African Swine Fever Virus Envelope Glycoprotein CD2v Interacts with Host CSF2RA to Regulate the JAK2-STAT3 Pathway and Inhibit Apoptosis to Facilitate Virus Replication
- PMID: 37022174
- PMCID: PMC10134862
- DOI: 10.1128/jvi.01889-22
African Swine Fever Virus Envelope Glycoprotein CD2v Interacts with Host CSF2RA to Regulate the JAK2-STAT3 Pathway and Inhibit Apoptosis to Facilitate Virus Replication
Abstract
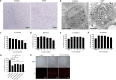
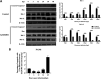
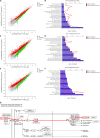
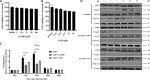
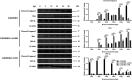
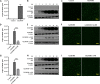
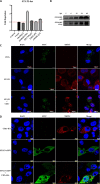
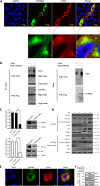
African swine fever (ASF) is a highly infectious disease caused by the African swine fever virus (ASFV) in swine. It is characterized by the death of cells in infected tissues. However, the molecular mechanism of ASFV-induced cell death in porcine alveolar macrophages (PAMs) remains largely unknown. In this study, transcriptome sequencing of ASFV-infected PAMs found that ASFV activated the JAK2-STAT3 pathway in the early stages and apoptosis in the late stages of infection. Meanwhile, the JAK2-STAT3 pathway was confirmed to be essential for ASFV replication. AG490 and andrographolide (AND) inhibited the JAK2-STAT3 pathway, promoted ASFV-induced apoptosis, and exerted antiviral effects. Additionally, CD2v promoted STAT3 transcription and phosphorylation as well as translocation into the nucleus. CD2v is the main envelope glycoprotein of the ASFV, and further investigations showed that CD2v deletion downregulates the JAK2-STAT3 pathway and promotes apoptosis to inhibit ASFV replication. Furthermore, we discovered that CD2v interacts with CSF2RA, which is a hematopoietic receptor superfamily member in myeloid cells and a key receptor protein that activates receptor-associated JAK and STAT proteins. In this study, CSF2RA small interfering RNA (siRNA) downregulated the JAK2-STAT3 pathway and promoted apoptosis to inhibit ASFV replication. Taken together, ASFV replication requires the JAK2-STAT3 pathway, while CD2v interacts with CSF2RA to regulate the JAK2-STAT3 pathway and inhibit apoptosis to facilitate virus replication. These results provide a theoretical basis for the escape mechanism and pathogenesis of ASFV. IMPORTANCE African swine fever is a hemorrhagic disease caused by the African swine fever virus (ASFV), which infects pigs of different breeds and ages, with a fatality rate of up to 100%. It is one of the key diseases affecting the global livestock industry. Currently, no commercial vaccines or antiviral drugs are available. Here, we show that ASFV replicates via the JAK2-STAT3 pathway. More specifically, ASFV CD2v interacts with CSF2RA to activate the JAK2-STAT3 pathway and inhibit apoptosis, thereby maintaining the survival of infected cells and promoting viral replication. This study revealed an important implication of the JAK2-STAT3 pathway in ASFV infection and identified a novel mechanism by which CD2v has evolved to interact with CSF2RA and maintain JAK2-STAT3 pathway activation to inhibit apoptosis, thus elucidating new information regarding the signal reprogramming of host cells by ASFV.
Keywords: ASFV; CD2v; CSF2RA; JAK2-STAT3 pathway; apoptosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Coreceptor AXL Facilitates African Swine Fever Virus Entry via Apoptotic Mimicry.J Virol. 2023 Jul 27;97(7):e0061623. doi: 10.1128/jvi.00616-23. Epub 2023 Jun 29. J Virol. 2023. PMID: 37382521 Free PMC article.
-
African Swine Fever Virus CD2v Protein Induces β-Interferon Expression and Apoptosis in Swine Peripheral Blood Mononuclear Cells.Viruses. 2021 Jul 28;13(8):1480. doi: 10.3390/v13081480. Viruses. 2021. PMID: 34452346 Free PMC article.
-
MGF360-9L Is a Major Virulence Factor Associated with the African Swine Fever Virus by Antagonizing the JAK/STAT Signaling Pathway.mBio. 2022 Feb 22;13(1):e0233021. doi: 10.1128/mbio.02330-21. Epub 2022 Jan 25. mBio. 2022. PMID: 35076286 Free PMC article.
-
African swine fever virus proteins involved in evading host defence systems.Vet Immunol Immunopathol. 2004 Aug;100(3-4):117-34. doi: 10.1016/j.vetimm.2004.04.002. Vet Immunol Immunopathol. 2004. PMID: 15207450 Review.
-
Structure of African Swine Fever Virus and Associated Molecular Mechanisms Underlying Infection and Immunosuppression: A Review.Front Immunol. 2021 Sep 6;12:715582. doi: 10.3389/fimmu.2021.715582. eCollection 2021. Front Immunol. 2021. PMID: 34552586 Free PMC article. Review.
Cited by
-
Punicalagin Inhibits African Swine Fever Virus Replication by Targeting Early Viral Stages and Modulating Inflammatory Pathways.Vet Sci. 2024 Sep 19;11(9):440. doi: 10.3390/vetsci11090440. Vet Sci. 2024. PMID: 39330819 Free PMC article.
-
Monoclonal Antibodies Targeting Porcine Macrophages Are Able to Inhibit the Cell Entry of Macrophage-Tropic Viruses (PRRSV and ASFV).Viruses. 2025 Jan 24;17(2):167. doi: 10.3390/v17020167. Viruses. 2025. PMID: 40006922 Free PMC article.
-
The Strategies Used by Animal Viruses to Antagonize Host Antiviral Innate Immunity: New Clues for Developing Live Attenuated Vaccines (LAVs).Vaccines (Basel). 2025 Jan 8;13(1):46. doi: 10.3390/vaccines13010046. Vaccines (Basel). 2025. PMID: 39852825 Free PMC article. Review.
-
STAT3 Increases CVB3 Replication and Acute Pancreatitis and Myocarditis Pathology via Impeding Nuclear Translocation of STAT1 and Interferon-Stimulated Gene Expression.Int J Mol Sci. 2024 Aug 19;25(16):9007. doi: 10.3390/ijms25169007. Int J Mol Sci. 2024. PMID: 39201692 Free PMC article.
-
Porcine alveolar macrophages host proteins interacting with African swine fever virus p72.Front Microbiol. 2024 Feb 28;15:1370417. doi: 10.3389/fmicb.2024.1370417. eCollection 2024. Front Microbiol. 2024. PMID: 38481793 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

