Take a Swipe at Actinide Bioavailability: Application of a New In Vitro Method
- PMID: 37022177
- PMCID: PMC10155695
- DOI: 10.1097/HP.0000000000001694
Take a Swipe at Actinide Bioavailability: Application of a New In Vitro Method
Abstract
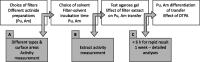
Filter swipe tests are used for routine analyses of actinides in nuclear industrial, research, and weapon facilities as well as following accidental release. Actinide physicochemical properties will determine in part bioavailability and internal contamination levels. The aim of this work was to develop and validate a new approach to predict actinide bioavailability recovered by filter swipe tests. As proof of concept and to simulate a routine or an accidental situation, filter swipes were obtained from a nuclear research facility glove box. A recently-developed biomimetic assay for prediction of actinide bioavailability was adapted for bioavailability measurements using material obtained from these filter swipes. In addition, the efficacy of the clinically-used chelator, diethylenetriamine pentaacetate (Ca-DTPA), to enhance transportability was determined. This report shows that it is possible to evaluate physicochemical properties and to predict bioavailability of filter swipe-associated actinides.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Health Physics Society.
Figures









References
-
- Aladova EE, Romanov SA, Guilmette RA, Khokhryakov VF, Suslova KG. In vitro dissolution study of plutonium in aerosol particles from the Mayak PA: a tool for individualised dose estimates. Radiat Protect Dosim 127:60–63; 2007. - PubMed
-
- André S, Charuau J, Rateau G, Vavasseur C, Métivier H. Design of a new inhalation device for rodents and primates. J Aerosol Sci 20:647-651, 653–656; 1989.
-
- Ansoborlo E, Hengé-Napoli MH, Chazel V, Gibert R, Guilmette RA. Review and critical analysis of available in vitro dissolution tests. Health Phys 77:638–645; 1999. - PubMed
-
- Bauer WF, Schuetz BK, Huestis GM, Lints TB, Harris BK, Duane Ball R, Elias G. In vitro dissolution tests of plutonium and americium containing contamination originating from ZPRR fuel plates. Idaho Falls, ID: Idaho National Laboratory; 2012. Available at https://inldigitallibrary.inl.gov/sites/sti/sti/5516329.pdf. Accessed 7 August 2022.
-
- Becková V, Malátová I. Dissolution behaviour of 238U, 234U and 230Th deposited on filters from personal dosemeters. Radiat Protect Dosim 129:469–472; 2008. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

