In Vitro FVIII-Encoding Transgenic Mesenchymal Stem Cells Maintain Successful Coagulation in FVIII-Deficient Plasma Mimicking Hemophilia A
- PMID: 37022209
- PMCID: PMC10240160
- DOI: 10.4274/tjh.galenos.2023.2022-0318
In Vitro FVIII-Encoding Transgenic Mesenchymal Stem Cells Maintain Successful Coagulation in FVIII-Deficient Plasma Mimicking Hemophilia A
Abstract
Objective: Hemophilia A is an X-linked recessive bleeding disorder caused by a deficiency of plasma coagulation factor VIII (FVIII), and it accounts for about 80%-85% of all cases of hemophilia. Plasma-derived therapies or recombinant FVIII concentrates are used to prevent and treat the bleeding symptoms along with FVIII-mimicking antibodies. Recently, the European Medicines Agency granted conditional marketing approval for the first gene therapy for hemophilia A. The aim of this study was to determine the effectiveness of coagulation in correcting FVIII deficiency with FVIII-secreting transgenic mesenchymal stem cells (MSCs).
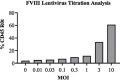
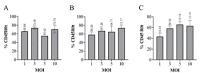
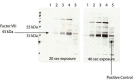
Materials and methods: A lentiviral vector encoding a B domain-deleted FVIII cDNA sequence with CD45R0 truncated (CD45R0t) surface marker was designed to develop a transgenic FVIII-expressing primary cell line by transducing MSCs. The efficacy and functionality of the FVIII secreted from the MSCs was assessed with anti-FVIII ELISA, CD45R0t flow cytometry, FVIII western blot, and mixing test analysis in vitro.
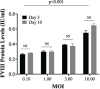
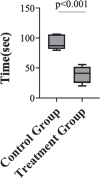
Results: The findings of this study showed that the transgenic MSCs maintained persistent FVIII secretion. There was no significant difference in FVIII secretion over time, suggesting stable FVIII expression from the MSCs. The functionality of the FVIII protein secreted in the MSC supernatant was demonstrated by applying a mixing test in coagulation analysis. In the mixing test analysis, FVIII-deficient human plasma products were mixed with either a saline control or FVIII-secreted MSC supernatant. The mean FVIII level of the saline control group was 0.41±0.03 IU/dL, whereas the mean level was 25.41±33.38 IU/dL in the FVIII-secreting MSC supernatant mixed group (p<0.01). The mean activated partial thromboplastin time (aPTT) of the saline control group was 92.69±11.38 s, while in the FVIII-secreting MSC supernatant mixed group, the mean aPTT level decreased to 38.60±13.38 s (p<0.001).
Conclusion: The findings of this in vitro study suggest that the new method presented here is promising as a possible treatment for hemophilia A. Accordingly, a study of FVIII-secreting transgenic MSCs will next be initiated in a FVIII-knockout animal model.
Amaç: Hemofili A, pıhtılaşma faktörü VIII’in (FVIII) eksikliğine bağlı gelişen, hemofili hastalarının yaklaşık %80-85’ini oluşturan, X’e bağlı resesif geçiş gösteren bir kanama bozukluğudur. Kanama semptomlarını önlemek ve tedavi etmek için plazma kaynaklı tedaviler ya da rekombinant FVIII konsantreleri ile FVIII’i taklit eden monoklonal antikorlar kullanılmaktadır. Son zamanlarda EMA, hemofili A’nın ilk gen tedavisi için koşullu pazarlama onayı vermiştir. Bu çalışmada, hemofili A hastalığından sorumlu olan FVIII eksikliğini düzeltebilmek amacıyla FVIII salgılayan transgenik mezenkimal kök hücreler (MKH) ile koagülasyon etkinliğinin değerlendirilmesi amaçlanmaktadır.
Gereç ve yöntemler: CD45R0 truncated (CD45R0t) yüzey belirteci ile B domaini silinmiş FVIII cDNA dizisini kodlayan bir lentiviral vektör, MKH’leri transdükte ederek transgenik FVIII ekspresyonu sağlayan birincil bir hücre hattı geliştirilmiştir. MKH’lerden salgılanan FVIII proteininin etkinliği ve fonksiyonalitesi anti-FVIII ELISA, CD45R0t akım sitometrisi, FVIII western blot ve karışım testi (mixing test) analizleri ile in vitro olarak değerlendirilmiştir.
Bulgular: Çalışmamızda transgenik MKH’lerin kalıcı FVIII sekresyonu sağladığı gösterilmiştir. Zamana bağlı yapılan analizlerde FVIII sekresyonunda anlamlı bir değişim olmadığı, MKH’lerde stabil FVIII ekspresyonu sağladığı gösterilmiştir. Koagülasyon analizlerinde, MKH süpernatanına sekrete olan FVIII proteininin fonksiyonalitesi, karışım testi kullanılarak saptanmıştır. Karışım testi analizlerinde, FVIII içermeyen insan plazma ürünleri salinle (kontrol grubu) ve FVIII-salgılayan MKH süpernatantı (çalışma grubu) ile karıştırılmıştır. Kontrol grubunun ortalama FVIII düzeylerinin 0,41±0,03 IU/dL olduğu, MKH süpernatant ile karıştırılan çalışma grubunda ise ortalama FVIII düzeylerinin 25,41±33,38 IU/dL (p<0,01) olduğu gösterilmiştir. Kontrol grubunun ortalama aktive parsiyel tromboplastin zamanı (aPTT) 92,69±11,38 saniye iken, FVIII-salgılayan MKH süpernatantı ile karıştırılan grubun ise ortalama aPTT seviyelerinin 38,60±13,38 saniyeye düştüğü gösterilmiştir (p<0,001).
Sonuç: Bu in vitro çalışma, hemofili A’yı tedavi etmek için uygulanabilir umut verici yeni bir yöntem ortaya koymaktadır. Bu veriler ışığında FVIII knock-out hayvan modelinde FVIII salgılayan transgenik MKH çalışması başlatılacaktır.
Keywords: Factor VIII; Gene therapy; Hemophilia A; Lentivirus; Mesenchymal stem cells.
©Copyright 2023 by Turkish Society of Hematology | Turkish Journal of Hematology, Published by Galenos Publishing House
Conflict of interest statement
Conflict of Interest: C.H., C.T., and E.O. are inventors of patent application (pending) including “Methods for the Production of Gene Products Suitable for Use in the Treatment of Hemophilia A” (2020/18990) at the Turkish Patent and Trademark Office. No other authors have a competing interests except for these authors.
Figures
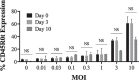

References
-
- Carcao MD. The diagnosis and management of congenital hemophilia. Semin Thromb Hemost. 2012;38:727–734. - PubMed
-
- Stonebraker JS, Bolton-Maggs PH, Soucie JM, Walker I, Brooker M. A study of variations in the reported haemophilia A prevalence around the world. Haemophilia. 2010;16:20–32. - PubMed
-
- Srivastava A, Santagostino E, Dougall A, Kitchen S, Sutherland M, Pipe SW, Carcao M, Mahlangu J, Ragni MV, Windyga J, Llinás A, Goddard NJ, Mohan R, Poonnoose PM, Feldman BM, Lewis SZ, van den Berg HM, Pierce GF; WFH Guidelines for the Management of Hemophilia panelists and co-authors. WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia. 2020;26 (Suppl 6):1–158. - PubMed
-
- White GC 2nd, Rosendaal F, Aledort LM, Lusher JM, Rothschild C, Ingerslev J; Factor VIII and Factor IX Subcommittee. Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 2001;85:560. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
