Evolutionarily Young African Rhinoceros Gammaretroviruses
- PMID: 37022231
- PMCID: PMC10134878
- DOI: 10.1128/jvi.01932-22
Evolutionarily Young African Rhinoceros Gammaretroviruses
Abstract
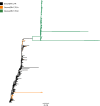
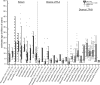
High-throughput sequences were generated from DNA and cDNA from four Southern white rhinoceros (Ceratotherium simum simum) located in the Taronga Western Plain Zoo in Australia. Virome analysis identified reads that were similar to Mus caroli endogenous gammaretrovirus (McERV). Previous analysis of perissodactyl genomes did not recover gammaretroviruses. Our analysis, including the screening of the updated white rhinoceros (Ceratotherium simum) and black rhinoceros (Diceros bicornis) draft genomes identified high-copy orthologous gammaretroviral ERVs. Screening of Asian rhinoceros, extinct rhinoceros, domestic horse, and tapir genomes did not identify related gammaretroviral sequences in these species. The newly identified proviral sequences were designated SimumERV and DicerosERV for the white and black rhinoceros retroviruses, respectively. Two long terminal repeat (LTR) variants (LTR-A and LTR-B) were identified in the black rhinoceros, with different copy numbers associated with each (n = 101 and 373, respectively). Only the LTR-A lineage (n = 467) was found in the white rhinoceros. The African and Asian rhinoceros lineages diverged approximately 16 million years ago. Divergence age estimation of the identified proviruses suggests that the exogenous retroviral ancestor of the African rhinoceros ERVs colonized their genomes within the last 8 million years, a result consistent with the absence of these gammaretroviruses from Asian rhinoceros and other perissodactyls. The black rhinoceros germ line was colonized by two lineages of closely related retroviruses and white rhinoceros by one. Phylogenetic analysis indicates a close evolutionary relationship with ERVs of rodents including sympatric African rats, suggesting a possible African origin of the identified rhinoceros gammaretroviruses. IMPORTANCE Rhinoceros genomes were thought to be devoid of gammaretroviruses, as has been determined for other perissodactyls (horses, tapirs, and rhinoceros). While this may be true of most rhinoceros, the African white and black rhinoceros genomes have been colonized by evolutionarily young gammaretroviruses (SimumERV and DicerosERV for the white and black rhinoceros, respectively). These high-copy endogenous retroviruses (ERVs) may have expanded in multiple waves. The closest relative of SimumERV and DicerosERV is found in rodents, including African endemic species. Restriction of the ERVs to African rhinoceros suggests an African origin for the rhinoceros gammaretroviruses.
Keywords: endogenous retrovirus; evolution; gammaretrovirus; perissodactyl; rhinoceros.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mayer J, Tsangaras K, Heeger F, Ávila-Arcos M, Stenglein MD, Chen W, Sun W, Mazzoni CJ, Osterrieder N, Greenwood AD. 2013. A novel endogenous betaretrovirus group characterized from polar bears (Ursus maritimus) and giant pandas (Ailuropoda melanoleuca). Virology 443:1–10. doi: 10.1016/j.virol.2013.05.008. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

