Tumour-derived extracellular vesicle based vaccines for melanoma treatment
- PMID: 37022605
- PMCID: PMC10102154
- DOI: 10.1007/s13346-023-01328-5
Tumour-derived extracellular vesicle based vaccines for melanoma treatment
Abstract
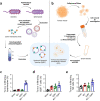
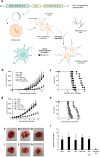
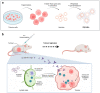
The interest of extracellular vesicles (EVs) in cancer immunotherapy is increasing every day. EVs are lipid bilayer vesicles released by most cells, which contain the molecular signature of their parent cell. Melanoma-derived EVs present antigens specific to this aggressive type of cancer, but they also exert immunomodulatory and pro-metastatic activity. Until now, most reviews focus on the immunoevasive characteristics of tumour-derived EVs, but do not help to overcome the issues related to them. In this review, we describe isolation methods of EVs from melanoma patients and most interesting markers to oversee their effect if they are used as antigen carriers. We also discuss the methods developed so far to overcome the lack of immunogenicity of melanoma-derived EVs, which includes EV modification or adjuvant co-administration. In summary, we conclude that EVs can be an interesting antigen source for immunotherapy development once EV obtaining is optimised and the understanding of the mechanisms behind their multiple effects is further understood.
Keywords: Exosomes; Extracellular vesicles; Immunostimulatory molecules; Immunotherapy; Melanoma; Tumour cells.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
The Roles of Extracellular Vesicles in Malignant Melanoma.Cells. 2021 Oct 14;10(10):2740. doi: 10.3390/cells10102740. Cells. 2021. PMID: 34685720 Free PMC article. Review.
-
An Immunocapture-Based Assay for Detecting Multiple Antigens in Melanoma-Derived Extracellular Vesicles.Methods Mol Biol. 2021;2265:323-344. doi: 10.1007/978-1-0716-1205-7_24. Methods Mol Biol. 2021. PMID: 33704725
-
Extracellular vesicles at the crossroad between cancer progression and immunotherapy: focus on dendritic cells.J Transl Med. 2024 Jul 29;22(1):691. doi: 10.1186/s12967-024-05457-4. J Transl Med. 2024. PMID: 39075551 Free PMC article. Review.
-
uPAR+ extracellular vesicles: a robust biomarker of resistance to checkpoint inhibitor immunotherapy in metastatic melanoma patients.J Immunother Cancer. 2021 May;9(5):e002372. doi: 10.1136/jitc-2021-002372. J Immunother Cancer. 2021. PMID: 33972390 Free PMC article.
-
Natural melanoma-derived extracellular vesicles.Semin Cancer Biol. 2019 Dec;59:251-265. doi: 10.1016/j.semcancer.2019.06.020. Epub 2019 Aug 3. Semin Cancer Biol. 2019. PMID: 31386906 Review.
Cited by
-
Tumor-associated macrophages as a potential therapeutic target in thyroid cancers.Cancer Immunol Immunother. 2023 Dec;72(12):3895-3917. doi: 10.1007/s00262-023-03549-6. Epub 2023 Oct 5. Cancer Immunol Immunother. 2023. PMID: 37796300 Free PMC article. Review.
-
Human Papillomavirus-Associated Tumor Extracellular Vesicles in HPV+ Tumor Microenvironments.J Clin Med. 2023 Aug 31;12(17):5668. doi: 10.3390/jcm12175668. J Clin Med. 2023. PMID: 37685735 Free PMC article. Review.
-
Tumor-derived exosomes and their application in cancer treatment.J Transl Med. 2025 Jul 8;23(1):751. doi: 10.1186/s12967-025-06814-7. J Transl Med. 2025. PMID: 40629355 Free PMC article. Review.
-
Grapefruit-Derived Vesicles Loaded with Recombinant HSP70 Activate Antitumor Immunity in Colon Cancer In Vitro and In Vivo.Biomedicines. 2024 Dec 3;12(12):2759. doi: 10.3390/biomedicines12122759. Biomedicines. 2024. PMID: 39767665 Free PMC article.
-
Unraveling the Multifaceted Roles of Extracellular Vesicles: Insights into Biology, Pharmacology, and Pharmaceutical Applications for Drug Delivery.Int J Mol Sci. 2023 Dec 29;25(1):485. doi: 10.3390/ijms25010485. Int J Mol Sci. 2023. PMID: 38203656 Free PMC article. Review.
References
-
- Cancer stat facts: melanoma of the skin. In: National Cancer Institute. https://seer.cancer.gov/statfacts/html/melan.html. Accessed 16 Jan 2023.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

