Efficacy and Safety of a Water-Free Topical Cyclosporine, 0.1%, Solution for the Treatment of Moderate to Severe Dry Eye Disease: The ESSENCE-2 Randomized Clinical Trial
- PMID: 37022717
- PMCID: PMC10080403
- DOI: 10.1001/jamaophthalmol.2023.0709
Efficacy and Safety of a Water-Free Topical Cyclosporine, 0.1%, Solution for the Treatment of Moderate to Severe Dry Eye Disease: The ESSENCE-2 Randomized Clinical Trial
Abstract
Importance: Dry eye disease (DED) is a common public health problem with significant impact on vision-related quality of life and well-being of patients. Medications with rapid onset of action and a good tolerability profile remain an unmet need.
Objective: To assess efficacy, safety, and tolerability of a water-free cyclosporine ophthalmic solution, 0.1% (CyclASol [Novaliq GmbH]), applied twice daily in DED compared with vehicle.
Design, setting, and participants: CyclASol for the Treatment of Signs and Symptoms of Dry Eye Disease (ESSENCE-2) was a phase 3, multicenter, randomized, double-masked, vehicle-controlled clinical study conducted from December 5, 2020, to October 8, 2021. Following a 14-day run-in period with an artificial tear administered 2 times per day, eligible participants were randomly assigned 1:1 to the treatment groups. Patients with moderate to severe DED were included in the study.
Interventions: Cyclosporine solution vs vehicle administered 2 times per day for 29 days.
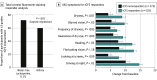
Main outcomes and measures: The primary end points were changes from baseline in total corneal fluorescein staining (tCFS; 0-15 National Eye Institute scale) and in dryness score (0-100 visual analog scale) at day 29. Conjunctival staining, central corneal fluorescein staining, and tCFS responders were also assessed.
Results: A total of 834 study participants were randomly assigned to cyclosporine (423 [50.7%]) or vehicle (411 [49.3%]) groups at 27 sites. Participants had a mean (SD) age of 57.1 (15.8) years, and 609 (73.0%) were female individuals. The majority of participants self-identified in the following race categories: 79 Asian (9.5 %), 108 Black (12.9%), and 635 White (76.1%). Participants treated with cyclosporine solution had greater improvement in tCFS (-4.0 grades) than the vehicle group (-3.6 grades) at day 29 (change [∆] = -0.4; 95% CI, -0.8 to 0; P = .03). The dryness score showed treatment benefits from baseline in both groups: -12.2 points for cyclosporine and -13.6 points for vehicle (∆ = 1.4; 95% CI, -1.8 to 4.6; P = .38). In the cyclosporine group, 293 participants (71.6%) achieved clinically meaningful reductions of 3 grades or higher in tCFS vs 236 (59.7%) in the vehicle group (∆ = 12.6%; 95% CI, 6.0%-19.3%; P < .001). These responders showed greater improvement in symptoms at day 29 including dryness (∆ = -4.6; 95% CI, -8.0 to -1.2; P = .007) and blurred vision (Δ = -3.5; 95% CI, -6.6 to -4.0; P = .03) compared with nonresponders.
Conclusions and relevance: The ESSENCE-2 trial confirmed that treatment with a water-free cyclosporine solution, 0.1%, results in early therapeutic effects on the ocular surface compared with vehicle. The responder analyses suggest that the effect is clinically meaningful in 71.6% of participants in the cyclosporine group.
Trial registration: ClinicalTrials.gov Identifier: NCT04523129.
Conflict of interest statement
Figures

Comment in
-
Comparison of Water-Free Commercially Available Cyclosporine Ophthalmic Preparations-Different, but the Same.JAMA Ophthalmol. 2023 May 1;141(5):466-467. doi: 10.1001/jamaophthalmol.2023.0850. JAMA Ophthalmol. 2023. PMID: 37022711 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

