Attenuation of Stimulated Accumbal Dopamine Release by NMDA Is Mediated through Metabotropic Glutamate Receptors
- PMID: 37022746
- PMCID: PMC10119936
- DOI: 10.1021/acschemneuro.2c00777
Attenuation of Stimulated Accumbal Dopamine Release by NMDA Is Mediated through Metabotropic Glutamate Receptors
Abstract
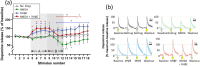
Electrically stimulated dopamine release from the nucleus accumbens is attenuated following application of N-methyl-d-aspartate (NMDA), which is likely to be mediated indirectly through intermediary neuronal mechanisms rather than by a direct action on dopamine terminals. On the basis of known modulatory processes in nucleus accumbens, the current experiments sought to test whether the effect of NMDA was mediated through cholinergic, GABA-ergic, or metabotropic glutamatergic intermediate mechanisms. Fast-scan cyclic voltammetry was used to measure electrically stimulated dopamine release in nucleus accumbens of rat brain slices in vitro. Stimulated dopamine release was attenuated by NMDA, confirming previous findings, but this attenuation was unaffected by either cholinergic or GABA-ergic antagonists. However, it was completely abolished by the nonselective group I/II/III metabotropic glutamate receptor antagonist α-methyl-4-carboxyphenylglycine (MCPG) and by the selective group II antagonist LY 341396. Therefore, group II metabotropic glutamate receptors, but not acetylcholine or GABA receptors, mediate the attenuation of stimulated dopamine release caused by NMDA, probably by presynaptic inhibition through receptors located extra-synaptically on dopamine terminals. This provides a plausible mechanism for the documented role of metabotropic glutamate receptor systems in restoring deficits induced by NMDA receptor antagonists, modeling schizophrenia, underlining the potential for drugs affecting these receptors as therapeutic agents in treating schizophrenia.
Keywords: Brain slices; N-methyl-d-aspartate (NMDA); dopamine; fast-scan cyclic voltammetry; metabotropic glutamate receptors; nucleus accumbens.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
N-Methyl-d-aspartate Modulation of Nucleus Accumbens Dopamine Release by Metabotropic Glutamate Receptors: Fast Cyclic Voltammetry Studies in Rat Brain Slices in Vitro.ACS Chem Neurosci. 2017 Feb 15;8(2):320-328. doi: 10.1021/acschemneuro.6b00397. Epub 2017 Feb 1. ACS Chem Neurosci. 2017. PMID: 28121123
-
Modulation of stimulated dopamine release in rat nucleus accumbens shell by GABA in vitro: Effect of sub-chronic phencyclidine pretreatment.J Neurosci Res. 2021 Jul;99(7):1885-1901. doi: 10.1002/jnr.24843. Epub 2021 Apr 13. J Neurosci Res. 2021. PMID: 33848365
-
Repeated phencyclidine disrupts nicotinic acetylcholine regulation of dopamine release in nucleus accumbens: Implications for models of schizophrenia.Neurochem Int. 2020 Nov;140:104836. doi: 10.1016/j.neuint.2020.104836. Epub 2020 Aug 24. Neurochem Int. 2020. PMID: 32853750
-
Excitatory amino acids: function and significance in reproduction and neuroendocrine regulation.Front Neuroendocrinol. 1994 Mar;15(1):3-49. doi: 10.1006/frne.1994.1002. Front Neuroendocrinol. 1994. PMID: 7958168 Review.
-
Magnesium in neuroses and neuroticism.In: Vink R, Nechifor M, editors. Magnesium in the Central Nervous System [Internet]. Adelaide (AU): University of Adelaide Press; 2011. In: Vink R, Nechifor M, editors. Magnesium in the Central Nervous System [Internet]. Adelaide (AU): University of Adelaide Press; 2011. PMID: 29920008 Free Books & Documents. Review.
Cited by
-
The dopaminergic effects of esketamine are mediated by a dual mechanism involving glutamate and opioid receptors.Mol Psychiatry. 2025 Aug;30(8):3443-3454. doi: 10.1038/s41380-025-02931-3. Epub 2025 Feb 19. Mol Psychiatry. 2025. PMID: 39972056 Free PMC article.
References
-
- Ayano G. Dopamine: receptors, functions, synthesis, pathways, locations and mental disorders: review of literatures. J. Ment. Disord. Treat. 2016, 2 (2), 1000120.10.4172/2471-271X.1000120. - DOI
LinkOut - more resources
Full Text Sources

