Size-dependent differences in mitochondrial volume density in phrenic motor neurons
- PMID: 37022966
- PMCID: PMC10190832
- DOI: 10.1152/japplphysiol.00021.2023
Size-dependent differences in mitochondrial volume density in phrenic motor neurons
Abstract
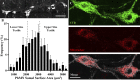
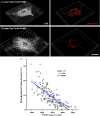
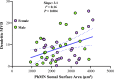
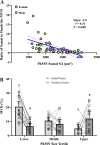
Neuromotor control of diaphragm muscle (DIAm) motor units is dependent on an orderly size-dependent recruitment of phrenic motor neurons (PhMNs). Slow (type S) and fast, fatigue resistant (type FR) DIAm motor units, which are frequently recruited to sustain ventilation, comprise smaller PhMNs that innervate type I and IIa DIAm fibers. More fatigable fast (type FF) motor units, which are infrequently recruited for higher force, expulsive behaviors, comprise larger PhMNs that innervate more type IIx/IIb DIAm fibers. We hypothesize that due to the more frequent activation and thus higher energy demand of type S and FR motor units, the mitochondrial volume density (MVD) of smaller PhMNs is greater compared with larger PhMNs. In eight adult (6 mo old) Fischer 344 rats, PhMNs were identified via intrapleural injection of Alexa488-conjugated cholera toxin B (CTB). Following retrograde CTB labeling, mitochondria in PhMNs were labeled by transdural infusion of MitoTracker Red. PhMNs and mitochondria were imaged using multichannel confocal microscopy using a ×60 oil objective. Following optical sectioning and three-dimensional (3-D) rendering, PhMNs and mitochondria were analyzed volumetrically using Nikon Elements software. Analysis of MVD in somal and dendritic compartments was stratified by PhMN somal surface area. Smaller PhMNs (likely S and FR units) had greater somal MVDs compared with larger PhMNs (likely FF units). By contrast, proximal dendrites or larger PhMNs had higher MVD compared with dendrites of smaller PhMNs. We conclude that more active smaller PhMNs have a higher mitochondrial volume density to support their higher energy demand in sustaining ventilation.NEW & NOTEWORTHY Type S and FR motor units, comprising smaller phrenic motor neurons (PhMNs) are regularly activated to perform indefatigable ventilatory requirements. By contrast, type FF motor units, comprising larger PhMNs, are infrequently activated to perform expulsive straining and airway defense maneuvers. This difference in activation history is mirrored in the mitochondrial volume density (MVD), with smaller PhMNs having higher MVD than larger PhMNs. In proximal dendrites, this trend was reversed, with larger PhMNs having higher MVD than smaller PhMNs, likely due to the maintenance requirements for the larger dendritic arbor of FF PhMNs.
Keywords: MitoTracker; MitoTracker Red; mitochondria; motor neurons.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

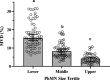
Similar articles
-
Aging affects the number and morphological heterogeneity of rat phrenic motor neurons and phrenic motor axons.Physiol Rep. 2023 Jan;11(2):e15587. doi: 10.14814/phy2.15587. Physiol Rep. 2023. PMID: 36695744 Free PMC article.
-
Phrenic motor neuron loss in aged rats.J Neurophysiol. 2018 May 1;119(5):1852-1862. doi: 10.1152/jn.00868.2017. Epub 2018 Feb 7. J Neurophysiol. 2018. PMID: 29412773 Free PMC article.
-
Glutamatergic input varies with phrenic motor neuron size.J Neurophysiol. 2019 Oct 1;122(4):1518-1529. doi: 10.1152/jn.00430.2019. Epub 2019 Aug 7. J Neurophysiol. 2019. PMID: 31389739 Free PMC article.
-
Phrenic motor unit recruitment during ventilatory and non-ventilatory behaviors.Respir Physiol Neurobiol. 2011 Oct 15;179(1):57-63. doi: 10.1016/j.resp.2011.06.028. Epub 2011 Jul 6. Respir Physiol Neurobiol. 2011. PMID: 21763470 Free PMC article. Review.
-
Convergence of pattern generator outputs on a common mechanism of diaphragm motor unit recruitment.Prog Brain Res. 2014;209:309-29. doi: 10.1016/B978-0-444-63274-6.00016-3. Prog Brain Res. 2014. PMID: 24746055 Free PMC article. Review.
References
-
- Sieck GC. Neural control of the inspiratory pump. Physiology 6: 260–264, 1991. doi:10.1152/physiologyonline.1991.6.6.260. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

