Maternal transfer of IgA and IgG SARS-CoV-2 specific antibodies transplacentally and via breast milk feeding
- PMID: 37023025
- PMCID: PMC10079052
- DOI: 10.1371/journal.pone.0284020
Maternal transfer of IgA and IgG SARS-CoV-2 specific antibodies transplacentally and via breast milk feeding
Abstract
Background: Although there have been many studies on antibody responses to SARS-CoV-2 in breast milk, very few have looked at the fate of these in the infant, and whether they are delivered to immunologically relevant sites in infants.
Methods: Mother/infant pairs (mothers who breast milk fed and who were SARS-CoV-2 vaccinated before or after delivery) were recruited for this cross-sectional study. Mother blood, mother breast milk, infant blood, infant nasal specimen, and infant stool was tested for IgA and IgG antibodies against SARS-CoV-2 spike trimer.
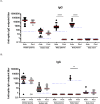
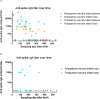
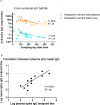
Results: Thirty-one mother/infant pairs were recruited. Breast milk fed infants acquired systemic anti-spike IgG antibodies only if their mothers were vaccinated antepartum (100% Antepartum; 0% Postpartum; P<0.0001). Breast milk fed infants acquired mucosal anti-spike IgG antibodies (in the nose) only if their mothers were vaccinated antepartum (89% Antepartum; 0% Postpartum; P<0.0001). None of the infants in either group had anti-spike IgA in the blood. Surprisingly, 33% of the infants whose mothers were vaccinated antepartum had high titer anti-spike IgA in the nose (33% Antepartum; 0% Postpartum; P = 0.03). Half-life of maternally transferred plasma IgG antibodies in the Antepartum infant cohort was ~70 days.
Conclusion: Vaccination antepartum followed by breast milk feeding appears to be the best way to provide systemic and local anti-SARS-CoV-2 antibodies for infants. The presence of high titer SARS-CoV-2-specific IgA in the nose of infants points to the potential importance of breast milk feeding early in life for maternal transfer of mucosal IgA antibodies. Expectant mothers should consider becoming vaccinated antepartum and consider breast milk feeding for optimal transfer of systemic and mucosal antibodies to their infants.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Royal College of Obstetricians and Gynaecologists. Coronavirus (COVID-19) Vaccination in Pregnancy., 2021).
-
- American College of Obstetricians and Gynecologists. Practice advisory: COVID-19 vaccination considerations for obstetric–gynecologic care., 2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

