Treatment pattern and clinical outcomes in multiple myeloma patients in Japan using the Medical Data Vision claims database
- PMID: 37023056
- PMCID: PMC10079007
- DOI: 10.1371/journal.pone.0283931
Treatment pattern and clinical outcomes in multiple myeloma patients in Japan using the Medical Data Vision claims database
Abstract
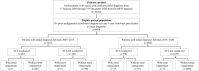
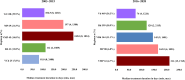
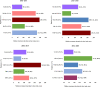
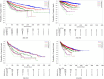
Multiple myeloma therapy has made remarkable progress with the advent of new drugs. We explored the treatment pattern and outcomes in Japanese patients with multiple myeloma using the Medical Data Vision database. Patients were categorized as per the initial diagnosis period (2003-2015 and 2016-2020), considering the adoption of these new agents and then based on stem cell transplantation. Overall, 6438 patient data were extracted as eligible for data analysis, and the median age at the index diagnosis date was 72.0 years. Bortezomib/dexamethasone was the most common regimen for induction therapy in patients requiring stem cell transplantation from 2003-2015, and the use of bortezomib/lenalidomide/dexamethasone increased from 2016-2020. Lenalidomide/dexamethasone was the most commonly used post-transplant therapy. In the non-stem cell transplantation group, bortezomib/dexamethasone was mainly used for both periods, while lenalidomide/dexamethasone was primarily used from 2016-2020. There was a trend toward shorter first-line treatment duration and a shift to additional treatment patterns with new drugs at the following lines. The time to inpatient death period suggested an improvement between the two periods. Thus, this study revealed that recent diversification of treatment options is preferred and contributes to improved outcomes in the clinical practice of multiple myeloma in Japan.
Copyright: © 2023 Handa et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests. HH received consulting fees and honoraria from Janssen, Ono, and Takeda; grants from Takeda, Kyowa Kirin, and BMS. TI received consulting fees and honoraria from Ono, Takeda, BMS, Janssen, and Sanofi; research fundings support from Takeda, Janssen, Pfizer, and BMS. SO has nothing to declare. AM is an employee of Janssen Pharmaceutical K.K. KK was an employee of Janssen Pharmaceutical K.K and is currently affiliated with Kite Japan Gilead Sciences, Tokyo, Japan, and have share in Gilead Science. SI is the current editor in Cancer Science journal and received consulting fees and honoraria from BMS, Janssen, Takeda, Ono, Sanofi, and Pfizer; research funding support from BMS, Janssen, Daiichi Sankyo, Amgen, Ono, AbbVie GK, GSK, Eli Lilly, Caelum Biosciences, Inc., Pfizer, Takeda, and Sanofi; grants from Chugai, Takeda, Ono, and Sanofi.
Figures

References
-
- National Cancer Registry (Ministry of Health, Labour and Welfare), tabulated by Cancer Information Service, National Cancer Center, Japan. (2016–2018), 2021. Accessed July 19, 2022. https://ganjoho.jp/reg_stat/statistics/stat/summary.html
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

