Translation of immunomodulatory therapy to treat chronic heart failure: Preclinical studies to first in human
- PMID: 37023139
- PMCID: PMC10079025
- DOI: 10.1371/journal.pone.0273138
Translation of immunomodulatory therapy to treat chronic heart failure: Preclinical studies to first in human
Abstract
Background: Inflammation has been associated with progression and complications of chronic heart failure (HF) but no effective therapy has yet been identified to treat this dysregulated immunologic state. The selective cytopheretic device (SCD) provides extracorporeal autologous cell processing to lessen the burden of inflammatory activity of circulating leukocytes of the innate immunologic system.
Aim: The objective of this study was to evaluate the effects of the SCD as an extracorporeal immunomodulatory device on the immune dysregulated state of HF. HF.
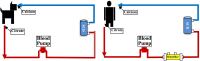
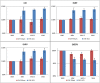
Methods and results: SCD treatment in a canine model of systolic HF or HF with reduced ejection fraction (HFrEF) diminished leukocyte inflammatory activity and enhanced cardiac performance as measured by left ventricular (LV) ejection fraction and stroke volume (SV) up to 4 weeks after treatment initiation. Translation of these observations in first in human, proof of concept clinical study was evaluated in a patient with severe HFrEFHFrEF ineligible for cardiac transplantation or LV LV assist device (LVAD) due to renal insufficiency and right ventricular dysfunction. Six hour SCD treatments over 6 consecutive days resulted in selective removal of inflammatory neutrophils and monocytes and reduction in key plasma cytokines, including tumor necrosis factor-alpha (TNF-α),), interleukin (IL)-6, IL-8, and monocyte chemoattractant protein (MCP)-1. These immunologic changes were associated with significant improvements in cardiac power output, right ventricular stroke work index, cardiac index and LVSV index…. Stabilization of renal function with progressive volume removal permitted successful LVAD implantation.
Conclusion: This translational research study demonstrates a promising immunomodulatory approach to improve cardiac performance in HFrEFHFrEF and supports the important role of inflammation in the progression of HFHF.
Copyright: © 2023 Humes et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: HDH, DAB, AJW: Innovative Biotherapies, Inc. and SeaStar Medical, Inc.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

