Increased Technology Use Associated With Lower A1C in a Large Pediatric Clinical Population
- PMID: 37023293
- PMCID: PMC10234743
- DOI: 10.2337/dc22-2121
Increased Technology Use Associated With Lower A1C in a Large Pediatric Clinical Population
Abstract
Objective: While continuous glucose monitors (CGMs), insulin pumps, and hybrid closed-loop (HCL) systems each improve glycemic control in type 1 diabetes, it is unclear how the use of these technologies impacts real-world pediatric care.
Research design and methods: We found 1,455 patients aged <22 years, with type 1 diabetes duration >3 months, and who had data from a single center in between both 2016-2017 (n = 2,827) and 2020-2021 (n = 2,731). Patients were grouped by multiple daily injections or insulin pump, with or without an HCL system, and using a blood glucose monitor or CGM. Glycemic control was compared using linear mixed-effects models adjusting for age, diabetes duration, and race/ethnicity.
Results: CGM use increased from 32.9 to 75.3%, and HCL use increased from 0.3 to 27.9%. Overall A1C decreased from 8.9 to 8.6% (P < 0.0001).
Conclusions: Adoption of CGM and HCL was associated with decreased A1C, suggesting promotion of these technologies may yield glycemic benefits.
© 2023 by the American Diabetes Association.
Conflict of interest statement
Figures


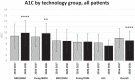
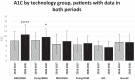
References
-
- de Bock M, Dart J, Hancock M, Smith G, Davis EA, Jones TW. Performance of Medtronic hybrid closed-loop iterations: results from a randomized trial in adolescents with type 1 diabetes. Diabetes Technol Ther 2018;20:693–697 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

