Porous Organic Cages
- PMID: 37023354
- PMCID: PMC10141292
- DOI: 10.1021/acs.chemrev.2c00667
Porous Organic Cages
Abstract
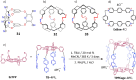
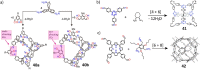
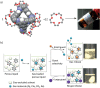
Porous organic cages (POCs) are a relatively new class of low-density crystalline materials that have emerged as a versatile platform for investigating molecular recognition, gas storage and separation, and proton conduction, with potential applications in the fields of porous liquids, highly permeable membranes, heterogeneous catalysis, and microreactors. In common with highly extended porous structures, such as metal-organic frameworks (MOFs), covalent organic frameworks (COFs), and porous organic polymers (POPs), POCs possess all of the advantages of highly specific surface areas, porosities, open pore channels, and tunable structures. In addition, they have discrete molecular structures and exhibit good to excellent solubilities in common solvents, enabling their solution dispersibility and processability─properties that are not readily available in the case of the well-established, insoluble, extended porous frameworks. Here, we present a critical review summarizing in detail recent progress and breakthroughs─especially during the past five years─of all the POCs while taking a close look at their strategic design, precise synthesis, including both irreversible bond-forming chemistry and dynamic covalent chemistry, advanced characterization, and diverse applications. We highlight representative POC examples in an attempt to gain some understanding of their structure-function relationships. We also discuss future challenges and opportunities in the design, synthesis, characterization, and application of POCs. We anticipate that this review will be useful to researchers working in this field when it comes to designing and developing new POCs with desired functions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures










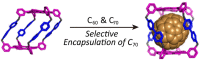


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

