Natural Cannabichromene (CBC) Shows Distinct Scalemicity Grades and Enantiomeric Dominance in Cannabis sativa Strains
- PMID: 37023389
- PMCID: PMC10152484
- DOI: 10.1021/acs.jnatprod.2c01139
Natural Cannabichromene (CBC) Shows Distinct Scalemicity Grades and Enantiomeric Dominance in Cannabis sativa Strains
Abstract
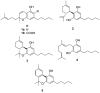
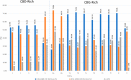
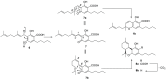
Cannabichromene (CBC, 1a) occurs in Cannabis (Cannabis sativa) as a scalemate having a composition that is strain-dependent in terms of both enantiomeric excess and enantiomeric dominance. In the present work, the chirality of CBC (1a), a noncrystalline compound, was shown not to be significantly affected by standard conditions of isolation and purification, and enantiomeric self-disproportionation effects were minimized by carrying out the chiral analysis on crude fractions rather than on purified products. A genetic basis for the different enantiomeric state of CBC in Cannabis therefore seems to exist, implying that the chirality status of natural CBC (1a) in the plant is associated with the differential expression of CBCA-synthase isoforms and/or of associated directing proteins with antipodal enantiospecificity. The biological profile of both enantiomers of CBC should therefore be investigated independently to assess the contribution of this compound to the activity of Cannabis preparations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

