The Adjuvant Combination of dmLT and Monophosphoryl Lipid A Activates the Canonical, Nonpyroptotic NLRP3 Inflammasome in Dendritic Cells and Significantly Interacts to Expand Antigen-Specific CD4 T Cells
- PMID: 37023458
- PMCID: PMC10159919
- DOI: 10.4049/jimmunol.2200221
The Adjuvant Combination of dmLT and Monophosphoryl Lipid A Activates the Canonical, Nonpyroptotic NLRP3 Inflammasome in Dendritic Cells and Significantly Interacts to Expand Antigen-Specific CD4 T Cells
Abstract
Adjuvants are often essential additions to vaccines that enhance the activation of innate immune cells, leading to more potent and protective T and B cell responses. Only a few vaccine adjuvants are currently used in approved vaccine formulations in the United States. Combinations of one or more adjuvants have the potential to increase the efficacy of existing and next-generation vaccines. In this study, we investigated how the nontoxic double mutant Escherichia coli heat-labile toxin R192G/L211A (dmLT), when combined with the TLR4 agonist monophosphoryl lipid A (MPL-A), impacted innate and adaptive immune responses to vaccination in mice. We found that the combination of dmLT and MPL-A induced an expansion of Ag-specific, multifaceted Th1/2/17 CD4 T cells higher than that explained by adding responses to either adjuvant alone. Furthermore, we observed more robust activation of primary mouse bone marrow-derived dendritic cells in the combination adjuvant-treated group via engagement of the canonical NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome complex. This was marked by a multiplicative increase in the secretion of active IL-1β that was independent of classical gasdermin D-mediated pyroptosis. Moreover, the combination adjuvant increased the production of the secondary messengers cAMP and PGE2 in dendritic cells. These results demonstrate how certain adjuvant combinations could be used to potentiate better vaccine responses to combat a variety of pathogens.
Copyright © 2023 by The American Association of Immunologists, Inc.
Conflict of interest statement
Figures
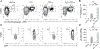
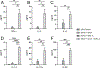
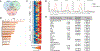
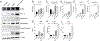

Similar articles
-
Identification of QS-21 as an Inflammasome-activating Molecular Component of Saponin Adjuvants.J Biol Chem. 2016 Jan 15;291(3):1123-36. doi: 10.1074/jbc.M115.683011. Epub 2015 Nov 10. J Biol Chem. 2016. PMID: 26555265 Free PMC article.
-
The adjuvant double mutant Escherichia coli heat labile toxin enhances IL-17A production in human T cells specific for bacterial vaccine antigens.PLoS One. 2012;7(12):e51718. doi: 10.1371/journal.pone.0051718. Epub 2012 Dec 20. PLoS One. 2012. PMID: 23284753 Free PMC article.
-
Effective Combination Adjuvants Engage Both TLR and Inflammasome Pathways To Promote Potent Adaptive Immune Responses.J Immunol. 2018 Jul 1;201(1):98-112. doi: 10.4049/jimmunol.1701604. Epub 2018 May 16. J Immunol. 2018. PMID: 29769270 Free PMC article.
-
The Mucosal Vaccine Adjuvant LT(R192G/L211A) or dmLT.mSphere. 2018 Jul 25;3(4):e00215-18. doi: 10.1128/mSphere.00215-18. mSphere. 2018. PMID: 30045966 Free PMC article. Review.
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
Cited by
-
Current vaccine strategies and novel approaches to combatting Francisella infection.Vaccine. 2024 Apr 2;42(9):2171-2180. doi: 10.1016/j.vaccine.2024.02.086. Epub 2024 Mar 8. Vaccine. 2024. PMID: 38461051 Free PMC article. Review.
-
Harnessing cellular immunity for next-generation vaccines against respiratory viruses: mechanisms, platforms, and optimization strategies.Front Immunol. 2025 Aug 13;16:1618406. doi: 10.3389/fimmu.2025.1618406. eCollection 2025. Front Immunol. 2025. PMID: 40881694 Free PMC article. Review.
-
Enterotoxigenic Escherichia coli heat labile enterotoxin affects neutrophil effector functions via cAMP/PKA/ERK signaling.Gut Microbes. 2024 Jan-Dec;16(1):2399215. doi: 10.1080/19490976.2024.2399215. Epub 2024 Sep 16. Gut Microbes. 2024. PMID: 39284098 Free PMC article.
-
Advances in vaccine adjuvant development and future perspectives.Drug Deliv. 2025 Dec;32(1):2517137. doi: 10.1080/10717544.2025.2517137. Epub 2025 Jun 19. Drug Deliv. 2025. PMID: 40536024 Free PMC article. Review.
-
Beyond Needles: Immunomodulatory Hydrogel-Guided Vaccine Delivery Systems.Gels. 2024 Dec 26;11(1):7. doi: 10.3390/gels11010007. Gels. 2024. PMID: 39851978 Free PMC article. Review.
References
-
- Michaud CM 2009. Encyclopedia of Microbiology (Third Edition). Pathogenesis Article Titles G 444–454.
-
- Doherty M, Buchy P, Standaert B, Giaquinto C, and Cohrs DP 2016. Vaccine impact: Benefits for human health. Vaccine 34: 6707–6714. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

