Long-term upper-extremity prosthetic control using regenerative peripheral nerve interfaces and implanted EMG electrodes
- PMID: 37023743
- PMCID: PMC10126717
- DOI: 10.1088/1741-2552/accb0c
Long-term upper-extremity prosthetic control using regenerative peripheral nerve interfaces and implanted EMG electrodes
Abstract
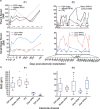
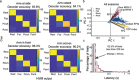
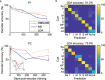
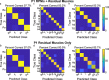
Objective.Extracting signals directly from the motor system poses challenges in obtaining both high amplitude and sustainable signals for upper-limb neuroprosthetic control. To translate neural interfaces into the clinical space, these interfaces must provide consistent signals and prosthetic performance.Approach.Previously, we have demonstrated that the Regenerative Peripheral Nerve Interface (RPNI) is a biologically stable, bioamplifier of efferent motor action potentials. Here, we assessed the signal reliability from electrodes surgically implanted in RPNIs and residual innervated muscles in humans for long-term prosthetic control.Main results.RPNI signal quality, measured as signal-to-noise ratio, remained greater than 15 for up to 276 and 1054 d in participant 1 (P1), and participant 2 (P2), respectively. Electromyography from both RPNIs and residual muscles was used to decode finger and grasp movements. Though signal amplitude varied between sessions, P2 maintained real-time prosthetic performance above 94% accuracy for 604 d without recalibration. Additionally, P2 completed a real-world multi-sequence coffee task with 99% accuracy for 611 d without recalibration.Significance.This study demonstrates the potential of RPNIs and implanted EMG electrodes as a long-term interface for enhanced prosthetic control.
Keywords: myoelectric control; neuroprosthetics; pattern recognition; peripheral nerve regeneration.
Creative Commons Attribution license.
Conflict of interest statement
All authors declare that they have no conflicts of interests. The University of Michigan holds a patent related to this work, publication number US10314725B2: Method for amplifying signals from individual nerve fascicles.
Figures


Similar articles
-
Use of regenerative peripheral nerve interfaces and intramuscular electrodes to improve prosthetic grasp selection: a case study.J Neural Eng. 2022 Nov 14;19(6):10.1088/1741-2552/ac9e1c. doi: 10.1088/1741-2552/ac9e1c. J Neural Eng. 2022. PMID: 36317254 Free PMC article. Clinical Trial.
-
A regenerative peripheral nerve interface allows real-time control of an artificial hand in upper limb amputees.Sci Transl Med. 2020 Mar 4;12(533):eaay2857. doi: 10.1126/scitranslmed.aay2857. Sci Transl Med. 2020. PMID: 32132217 Free PMC article.
-
Regenerative peripheral nerve interfaces for real-time, proportional control of a Neuroprosthetic hand.J Neuroeng Rehabil. 2018 Nov 20;15(1):108. doi: 10.1186/s12984-018-0452-1. J Neuroeng Rehabil. 2018. PMID: 30458876 Free PMC article.
-
Regenerative Peripheral Nerve Interfaces for Advanced Control of Upper Extremity Prosthetic Devices.Hand Clin. 2021 Aug;37(3):425-433. doi: 10.1016/j.hcl.2021.04.005. Hand Clin. 2021. PMID: 34253315 Review.
-
Targeted Muscle Reinnervation and Regenerative Peripheral Nerve Interface for Myoelectric Prosthesis Control: The State of Evidence.Ann Plast Surg. 2025 Jun 1;94(6S Suppl 4):S572-S576. doi: 10.1097/SAP.0000000000004273. Ann Plast Surg. 2025. PMID: 40459463 Review.
Cited by
-
Selective peripheral nerve recording using simulated human median nerve activity and convolutional neural networks.Biomed Eng Online. 2023 Dec 7;22(1):118. doi: 10.1186/s12938-023-01181-0. Biomed Eng Online. 2023. PMID: 38062509 Free PMC article.
-
Enhancing neuroprosthesis calibration: the advantage of integrating prior training over exclusive use of new data.J Neural Eng. 2024 Nov 29;21(6):066020. doi: 10.1088/1741-2552/ad94a7. J Neural Eng. 2024. PMID: 39569866 Free PMC article.
-
The Next Frontier in Neuroprosthetics: Integration of Biomimetic Somatosensory Feedback.Biomimetics (Basel). 2025 Feb 21;10(3):130. doi: 10.3390/biomimetics10030130. Biomimetics (Basel). 2025. PMID: 40136784 Free PMC article. Review.
-
Biocompatible Electrical and Optical Interfaces for Implantable Sensors and Devices.Sensors (Basel). 2024 Jun 12;24(12):3799. doi: 10.3390/s24123799. Sensors (Basel). 2024. PMID: 38931581 Free PMC article. Review.
-
Beyond Amputation: Functional Restoration after Upper-Extremity Limb Loss.J Hand Surg Glob Online. 2025 Feb 7;7(2):368-375. doi: 10.1016/j.jhsg.2025.01.008. eCollection 2025 Mar. J Hand Surg Glob Online. 2025. PMID: 40182885 Free PMC article.
References
-
- Østlie K, Lesjø I M, Franklin R J, Garfelt B, Skjeldal O H, Magnus P. Prosthesis use in adult acquired major upper-limb amputees: patterns of wear, prosthetic skills and the actual use of prostheses in activities of daily life. Disabil. Rehabil. Assist. Technol. 2012;7:479–93. doi: 10.3109/17483107.2011.653296. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
