Benefits and harms of drug treatment for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials
- PMID: 37024129
- PMCID: PMC10077111
- DOI: 10.1136/bmj-2022-074068
Benefits and harms of drug treatment for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials
Abstract
Objective: To compare the benefits and harms of drug treatments for adults with type 2 diabetes, adding non-steroidal mineralocorticoid receptor antagonists (including finerenone) and tirzepatide (a dual glucose dependent insulinotropic polypeptide (GIP)/glucagon-like peptide-1 (GLP-1) receptor agonist) to previously existing treatment options.
Design: Systematic review and network meta-analysis.
Data sources: Ovid Medline, Embase, and Cochrane Central up to 14 October 2022.
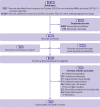
Eligibility criteria for selecting studies: Eligible randomised controlled trials compared drugs of interest in adults with type 2 diabetes. Eligible trials had a follow-up of 24 weeks or longer. Trials systematically comparing combinations of more than one drug treatment class with no drug, subgroup analyses of randomised controlled trials, and non-English language studies were deemed ineligible. Certainty of evidence was assessed following the GRADE (grading of recommendations, assessment, development and evaluation) approach.
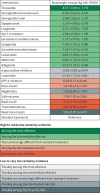
Results: The analysis identified 816 trials with 471 038 patients, together evaluating 13 different drug classes; all subsequent estimates refer to the comparison with standard treatments. Sodium glucose cotransporter-2 (SGLT-2) inhibitors (odds ratio 0.88, 95% confidence interval 0.83 to 0.94; high certainty) and GLP-1 receptor agonists (0.88, 0.82 to 0.93; high certainty) reduce all cause death; non-steroidal mineralocorticoid receptor antagonists, so far tested only with finerenone in patients with chronic kidney disease, probably reduce mortality (0.89, 0.79 to 1.00; moderate certainty); other drugs may not. The study confirmed the benefits of SGLT-2 inhibitors and GLP-1 receptor agonists in reducing cardiovascular death, non-fatal myocardial infarction, admission to hospital for heart failure, and end stage kidney disease. Finerenone probably reduces admissions to hospital for heart failure and end stage kidney disease, and possibly cardiovascular death. Only GLP-1 receptor agonists reduce non-fatal stroke; SGLT-2 inhibitors are superior to other drugs in reducing end stage kidney disease. GLP-1 receptor agonists and probably SGLT-2 inhibitors and tirzepatide improve quality of life. Reported harms were largely specific to drug class (eg, genital infections with SGLT-2 inhibitors, severe gastrointestinal adverse events with tirzepatide and GLP-1 receptor agonists, hyperkalaemia leading to admission to hospital with finerenone). Tirzepatide probably results in the largest reduction in body weight (mean difference -8.57 kg; moderate certainty). Basal insulin (mean difference 2.15 kg; moderate certainty) and thiazolidinediones (mean difference 2.81 kg; moderate certainty) probably result in the largest increases in body weight. Absolute benefits of SGLT-2 inhibitors, GLP-1 receptor agonists, and finerenone vary in people with type 2 diabetes, depending on baseline risks for cardiovascular and kidney outcomes (https://matchit.magicevidence.org/230125dist-diabetes).
Conclusions: This network meta-analysis extends knowledge beyond confirming the substantial benefits with the use of SGLT-2 inhibitors and GLP-1 receptor agonists in reducing adverse cardiovascular and kidney outcomes and death by adding information on finerenone and tirzepatide. These findings highlight the need for continuous assessment of scientific progress to introduce cutting edge updates in clinical practice guidelines for people with type 2 diabetes.
Systematic review registration: PROSPERO CRD42022325948.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: support from Sichuan Science and Technology Bureau and West China Hospital, Sichuan University for the submitted work; QS, KNo, POV, AAg, TA, RS, QH, QF, ZQ, FY, XZ, XC, YJ, LG, YM, QinY, AAs, CZ, JPL, KNu, SRC, SG, YG, XL, QiuY, HZ, XA, ZC, XL, SH, YC, HT, and GHG received no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. ES reported personal fees from Oxford Diabetes Trials Unit, Bayer, Berlin Chemie, Boehringer Ingelheim, Menarini, Merck Serono, EXCEMED, Novartis, Novo Nordisk, and Sanofi. LR reported grants or contracts from Swedish Heart Lung Foundation, Stockholm County Council, Erling Perssons Foundation, and Boehringer-Ingelheim, and payment or honorariums for lectures, presentations, speakers bureaus, manuscript writing or educational events from Bayer AG, Boehringer Ingelheim, and Novo Nordisk. FCB reported grants or contracts from National Institutes of Health, and consulting fees from Gilead Sciences. RAM reported grants or contracts from Boehringer Ingelheim. OS reported payment or honorariums for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Abbott Diagnostics, Lilly Deutschland, Boehringer Ingelheim, Bayer, Mannkind, and Lifescan and is a founder and CEO of Sciarc GmbH. NM reported grants or contracts from Boehringer Ingelheim, Merck, Novo Nordisk, Deutsche Forschungsgesellschaft (German Research Foundation; TRR 219), and consulting fees from Boehringer Ingelheim, Merck, Novo Nordisk, AstraZeneca, BMS, and payment or honorariums for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Boehringer Ingelheim, Merck, Novo Nordisk, Lilly, BMS, and AstraZeneca. SL received the fund from the Sichuan Science and Technology Programme and West China Hospital of Sichuan University.
Figures



References
-
- Gregg EW, Jakicic JM, Blackburn G, et al. Look AHEAD Research Group . Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2016;4:913-21. 10.1016/S2213-8587(16)30162-0. - DOI - PMC - PubMed
-
- Palmer SC, Tendal B, Mustafa RA, et al. . Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ 2021;372:m4573. 10.1136/bmj.m4573. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
