MELAS: Phenotype Classification into Classic-versus-Atypical Presentations
- PMID: 37024306
- PMCID: PMC10171385
- DOI: 10.3174/ajnr.A7837
MELAS: Phenotype Classification into Classic-versus-Atypical Presentations
Abstract
Background and purpose: An increased number of pathogenic variants have been described in mitochondrial encephalomyopathy lactic acidosis and strokelike episodes (MELAS). Different imaging presentations have emerged in parallel with a growing recognition of clinical and outcome variability, which pose a diagnostic challenge to neurologists and radiologists and may impact an individual patient's response to therapeutic interventions. By evaluating clinical, neuroimaging, laboratory, and genetic findings, we sought to improve our understanding of the sources of potential phenotype variability in patients with MELAS.
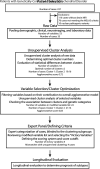
Materials and methods: This retrospective single-center study included individuals who had confirmed mitochondrial DNA pathogenic variants and a diagnosis of MELAS and whose data were reviewed from January 2000 through November 2021. The approach included a review of clinical, neuroimaging, laboratory, and genetic data, followed by an unsupervised hierarchical cluster analysis looking for sources of phenotype variability in MELAS. Subsequently, experts identified "victory-variables" that best differentiated MELAS cohort clusters.
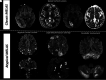
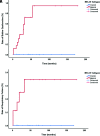
Results: Thirty-five patients with a diagnosis of mitochondrial DNA-based MELAS (median age, 12 years; interquartile range, 7-24 years; 24 female) were eligible for this study. Fifty-three discrete variables were evaluated by an unsupervised cluster analysis, which revealed that two distinct phenotypes exist among patients with MELAS. After experts reviewed the variables, they selected 8 victory-variables with the greatest impact in determining the MELAS subgroups: developmental delay, sensorineural hearing loss, vision loss in the first strokelike episode, Leigh syndrome overlap, age at the first strokelike episode, cortical lesion size, regional brain distribution of lesions, and genetic groups. Ultimately, 2-step differentiating criteria were defined to classify atypical MELAS.
Conclusions: We identified 2 distinct patterns of MELAS: classic MELAS and atypical MELAS. Recognizing different patterns in MELAS presentations will enable clinical and research care teams to better understand the natural history and prognosis of MELAS and identify the best candidates for specific therapeutic interventions.
© 2023 by American Journal of Neuroradiology.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
