Chikungunya fever
- PMID: 37024497
- PMCID: PMC11126297
- DOI: 10.1038/s41572-023-00429-2
Chikungunya fever
Erratum in
-
Author Correction: Chikungunya fever.Nat Rev Dis Primers. 2023 May 19;9(1):26. doi: 10.1038/s41572-023-00442-5. Nat Rev Dis Primers. 2023. PMID: 37208376 No abstract available.
Abstract
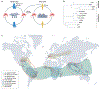
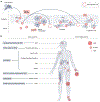
Chikungunya virus is widespread throughout the tropics, where it causes recurrent outbreaks of chikungunya fever. In recent years, outbreaks have afflicted populations in East and Central Africa, South America and Southeast Asia. The virus is transmitted by Aedes aegypti and Aedes albopictus mosquitoes. Chikungunya fever is characterized by severe arthralgia and myalgia that can persist for years and have considerable detrimental effects on health, quality of life and economic productivity. The effects of climate change as well as increased globalization of commerce and travel have led to growth of the habitat of Aedes mosquitoes. As a result, increasing numbers of people will be at risk of chikungunya fever in the coming years. In the absence of specific antiviral treatments and with vaccines still in development, surveillance and vector control are essential to suppress re-emergence and epidemics.
© 2023. Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures



References
-
- Kramer IM et al. The ecophysiological plasticity of Aedes aegypti and Aedes albopictus concerning overwintering in cooler ecoregions is driven by local climate and acclimation capacity. Sci. Total. Environ 778, 146128 (2021). - PubMed
-
- Zaid A et al. Arthritogenic alphaviruses: epidemiological and clinical perspective on emerging arboviruses. Lancet Infect. Dis 21, e123–e133 (2021) - PubMed
-
This review focuses on CHIKV and other arthritogenic alphaviruses that have been identified globally, and provides a comprehensive appraisal of present and future research directions.
-
- Weaver SC, Chen R & Diallo M Chikungunya virus: role of vectors in emergence from enzootic cycles. Annu. Rev. Entomol 65, 313–332 (2020). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

