Tumor inflammation-associated neurotoxicity
- PMID: 37024595
- PMCID: PMC10166099
- DOI: 10.1038/s41591-023-02276-w
Tumor inflammation-associated neurotoxicity
Abstract
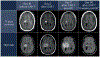
Cancer immunotherapies have unique toxicities. Establishment of grading scales and standardized grade-based treatment algorithms for toxicity syndromes can improve the safety of these treatments, as observed for cytokine release syndrome (CRS) and immune effector cell associated neurotoxicity syndrome (ICANS) in patients with B cell malignancies treated with chimeric antigen receptor (CAR) T cell therapy. We have observed a toxicity syndrome, distinct from CRS and ICANS, in patients treated with cell therapies for tumors in the central nervous system (CNS), which we term tumor inflammation-associated neurotoxicity (TIAN). Encompassing the concept of 'pseudoprogression,' but broader than inflammation-induced edema alone, TIAN is relevant not only to cellular therapies, but also to other immunotherapies for CNS tumors. To facilitate the safe administration of cell therapies for patients with CNS tumors, we define TIAN, propose a toxicity grading scale for TIAN syndrome and discuss the potential management of this entity, with the goal of standardizing both reporting and management.
© 2023. Springer Nature America, Inc.
Conflict of interest statement
Competing interests
C.L.M. holds multiple patents in the arena of CAR T cell therapeutics, is a cofounder and holds equity in Lyell Immunopharma, CARGO Therapeutics and Link Cell Therapies, which are developing CAR-based therapies, and consults for Lyell, CARGO, Link, NeoImmune Tech, Apricity, Nektar, Immatics, Mammoth and Ensoma. R.G.M. holds patents for CAR T cell therapeutics and is a cofounder of and holds equity in CARGO Therapeutics and Link Cell Therapies. R.G.M. has served as a consultant for Lyell Immunopharma, CARGO Therapeutics, Link Cell Therapies, NKarta, Arovella Pharmaceuticals, ImmunAI, Aptorum Group, Zai Labs, Innervate Radiopharmaceuticals, GaDeta and GammaDelta Therapeutics. J.G. is a consultant for Johnson & Johnson. J.D. has been a consultant for Amgen and Unum Therapeutics. M.M. holds patents for CAR T cell therapeutics and holds equity in MapLight Therapeutics. M.L. receives research support from Arbor, BMS, Accuray, Biohaven and Urogen; serves as a consultant to VBI, InCephalo Therapeutics, Merck, Pyramid Bio, Insightec, Biohaven, Sanianoia, Hemispherian, Novocure, Noxxon, InCando, Century Therapeutics, CraniUs, MediFlix and XSense; and is a shareholder in Egret Therapeutics.
Figures
References
-
- Medikonda R, Dunn G, Rahman M, Fecci P & Lim M A review of glioblastoma immunotherapy. J. Neurooncol 151, 41–53 (2021). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

