A C-H activation-based enantioselective synthesis of lower carbo[n]helicenes
- PMID: 37024717
- PMCID: PMC10239729
- DOI: 10.1038/s41557-023-01174-5
A C-H activation-based enantioselective synthesis of lower carbo[n]helicenes
Abstract
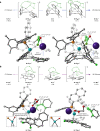
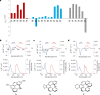
The three-dimensional structure of carbohelicenes has fascinated generations of molecular chemists and has been exploited in a wide range of applications. Their strong circularly polarized luminescence has attracted considerable attention in recent years due to promising applications in new optical materials. Although the enantioselective synthesis of fused carbo- and heterohelicenes has been achieved, a direct catalytic enantioselective method allowing the synthesis of lower, non-fused carbo[n]helicenes (n = 4-6) is still lacking. We report here that Pd-catalysed enantioselective C-H arylation in the presence of a unique bifunctional phosphine-carboxylate ligand provides a simple and general access to these lower carbo[n]helicenes. Computational mechanistic studies indicate that both the C-H activation and reductive elimination steps contribute to the overall enantioselectivity. The observed enantio-induction seems to arise from a combination of non-covalent interactions and steric repulsion between the substrate and ligand during the two key reductive elimination steps. The photophysical and chiroptical properties of the synthesized scalemic [n]helicenes have been systematically studied.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Chen, C. F. & Shen, Y. Helicene Chemistry: From Synthesis to Applications (Springer, 2017).
-
- Ravat P, Šolomek T, Juríček M. Helicenes as chiroptical photoswitches. ChemPhotoChem. 2019;3:180–186. doi: 10.1002/cptc.201800229. - DOI
LinkOut - more resources
Full Text Sources

