The GAPDH redox switch safeguards reductive capacity and enables survival of stressed tumour cells
- PMID: 37024754
- PMCID: PMC10132988
- DOI: 10.1038/s42255-023-00781-3
The GAPDH redox switch safeguards reductive capacity and enables survival of stressed tumour cells
Abstract
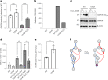
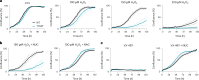
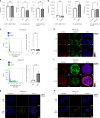
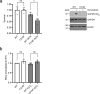
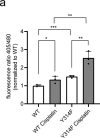
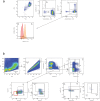
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is known to contain an active-site cysteine residue undergoing oxidation in response to hydrogen peroxide, leading to rapid inactivation of the enzyme. Here we show that human and mouse cells expressing a GAPDH mutant lacking this redox switch retain catalytic activity but are unable to stimulate the oxidative pentose phosphate pathway and enhance their reductive capacity. Specifically, we find that anchorage-independent growth of cells and spheroids is limited by an elevation of endogenous peroxide levels and is largely dependent on a functional GAPDH redox switch. Likewise, tumour growth in vivo is limited by peroxide stress and suppressed when the GAPDH redox switch is disabled in tumour cells. The induction of additional intratumoural oxidative stress by chemo- or radiotherapy synergized with the deactivation of the GAPDH redox switch. Mice lacking the GAPDH redox switch exhibit altered fatty acid metabolism in kidney and heart, apparently in compensation for the lack of the redox switch. Together, our findings demonstrate the physiological and pathophysiological relevance of oxidative GAPDH inactivation in mammals.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







Comment in
-
GAPDH redox redux-rewiring pentose phosphate flux.Nat Metab. 2023 Apr;5(4):538-539. doi: 10.1038/s42255-021-00523-3. Nat Metab. 2023. PMID: 37024755 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

