Efficacy and safety of transurethral resection of bladder tumour combined with chemotherapy and immunotherapy in bladder-sparing therapy in patients with T1 high-grade or T2 bladder cancer: a protocol for a randomized controlled trial
- PMID: 37024824
- PMCID: PMC10080830
- DOI: 10.1186/s12885-023-10798-2
Efficacy and safety of transurethral resection of bladder tumour combined with chemotherapy and immunotherapy in bladder-sparing therapy in patients with T1 high-grade or T2 bladder cancer: a protocol for a randomized controlled trial
Abstract
Background: Bladder cancer is the tenth most common cancer worldwide. For patients with T1 high-grade or T2 bladder cancer, radical cystectomy is recommended. However, radical cystectomy is associated with various complications and has a detrimental impact on the quality of life. Bladder-sparing therapy has been widely explored in patients with muscle-invasive bladder cancer, and whether a combination of transurethral resection of bladder tumour (TURBT) with chemotherapy and immunotherapy shows definite superiority over TURBT plus chemotherapy is still a matter of debate. The aim of this study is to investigate the efficacy and safety of TURBT combined with chemotherapy and immunotherapy in bladder-sparing therapy in patients with T1 high-grade or T2 bladder cancer who are unwilling or unsuitable to undergo radical cystectomy.
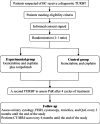
Methods: An open-label, multi-institutional, two-armed randomized controlled study will be performed with 86 patients with T1 high-grade or T2 bladder cancer meeting the eligibility criteria. Participants in the experimental group (n = 43) will receive TURBT combined with chemotherapy (GC: gemcitabine 1000 mg/m2 on the 1st day and the 8th day, cisplatin 70 mg/m2 on the 2nd day, repeated every 21 days) and immunotherapy (toripalimab 240 mg on the 5th day, repeated every 21 days), and those in the control group (n = 43) will receive TURBT plus chemotherapy (GC). The primary outcome is pathological response, and the secondary outcomes include progression-free survival, overall survival, toxicities, and quality of life.
Discussion: To the best of our knowledge, this is the first study to evaluate the efficacy and safety of TURBT combined with GC regimen and toripalimab in bladder-sparing therapy in patients with T1 high-grade or T2 bladder cancer. The expected benefit is that the combination of TURBT with chemotherapy and immunotherapy would be more effective than TURBT plus chemotherapy without compromising the quality of life and increasing the toxicity.
Trial registration: ChiCTR2200060546, chictr.org.cn, registered on June 14, 2022.
Keywords: Bladder cancer; Bladder-sparing therapy; Chemotherapy; Efficacy; Immunotherapy; Safety; Transurethral resection of bladder tumour.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Feasibility and Oncological Outcome of Patients Achieving Noninvasive Downstaging After Transurethral Resection of Bladder Tumor Plus Systemic Chemotherapy for Bladder Preservation Strategy in Muscle-Invasive Bladder Cancer.Clin Genitourin Cancer. 2025 Apr;23(2):102290. doi: 10.1016/j.clgc.2024.102290. Epub 2024 Dec 10. Clin Genitourin Cancer. 2025. PMID: 39798182
-
Impact of noninvasive down-staging after transurethral resection of bladder tumor plus systemic chemotherapy on bladder-sparing strategy in patients with muscle-invasive bladder cancer.Urol Oncol. 2021 Feb;39(2):132.e1-132.e6. doi: 10.1016/j.urolonc.2020.07.016. Epub 2020 Aug 10. Urol Oncol. 2021. PMID: 32792215
-
Prevalence and outcomes of transurethral resection versus radical cystectomy for muscle-infiltrating bladder cancer in the United States: A population-based cohort study.Int J Surg. 2022 Jul;103:106693. doi: 10.1016/j.ijsu.2022.106693. Epub 2022 Jun 9. Int J Surg. 2022. PMID: 35690361
-
Management of high-risk non-muscle invasive bladder cancer.Minerva Urol Nefrol. 2012 Dec;64(4):255-60. Minerva Urol Nefrol. 2012. PMID: 23288212 Review.
-
Treatment strategies using transurethral surgery, chemotherapy, and radiation therapy with selection that safely allows bladder conservation for invasive bladder cancer.Semin Surg Oncol. 1997 Sep-Oct;13(5):359-64. doi: 10.1002/(sici)1098-2388(199709/10)13:5<359::aid-ssu10>3.0.co;2-i. Semin Surg Oncol. 1997. PMID: 9259092 Review.
Cited by
-
Treatment Patterns and Radical Cystectomy Outcomes in Patients Diagnosed With Urothelial Nonmetastatic Muscle-Invasive Bladder Cancer in the United States.Cancer Med. 2025 Feb;14(4):e70644. doi: 10.1002/cam4.70644. Cancer Med. 2025. PMID: 39945337 Free PMC article.
-
SRT3025-loaded cell membrane hybrid liposomes (3025@ML) enhanced anti-tumor activity of Oxaliplatin via inhibiting pyruvate kinase M2 and fatty acid synthase.Lipids Health Dis. 2025 Jan 17;24(1):14. doi: 10.1186/s12944-025-02431-x. Lipids Health Dis. 2025. PMID: 39825408 Free PMC article.
-
Evaluating the efficacy and safety of bladder-sparing regimen with Disitamab Vedotin combined with Toripalimab and pelvic lymph node dissection in muscle-invasive bladder cancer patients: study protocol of a multicenter single-arm phase II trial.BMC Cancer. 2025 May 13;25(1):868. doi: 10.1186/s12885-025-14234-5. BMC Cancer. 2025. PMID: 40361038 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous