Chenodeoxycholic acid rescues axonal degeneration in induced pluripotent stem cell-derived neurons from spastic paraplegia type 5 and cerebrotendinous xanthomatosis patients
- PMID: 37024986
- PMCID: PMC10080795
- DOI: 10.1186/s13023-023-02666-w
Chenodeoxycholic acid rescues axonal degeneration in induced pluripotent stem cell-derived neurons from spastic paraplegia type 5 and cerebrotendinous xanthomatosis patients
Abstract
Background: Biallelic mutations in CYP27A1 and CYP7B1, two critical genes regulating cholesterol and bile acid metabolism, cause cerebrotendinous xanthomatosis (CTX) and hereditary spastic paraplegia type 5 (SPG5), respectively. These rare diseases are characterized by progressive degeneration of corticospinal motor neuron axons, yet the underlying pathogenic mechanisms and strategies to mitigate axonal degeneration remain elusive.
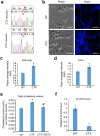
Methods: To generate induced pluripotent stem cell (iPSC)-based models for CTX and SPG5, we reprogrammed patient skin fibroblasts into iPSCs by transducing fibroblast cells with episomal vectors containing pluripotency factors. These patient-specific iPSCs, as well as control iPSCs, were differentiated into cortical projection neurons (PNs) and examined for biochemical alterations and disease-related phenotypes.
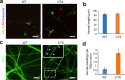
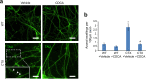
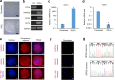
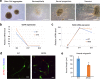
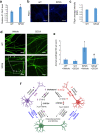
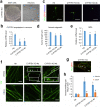
Results: CTX and SPG5 patient iPSC-derived cortical PNs recapitulated several disease-specific biochemical changes and axonal defects of both diseases. Notably, the bile acid chenodeoxycholic acid (CDCA) effectively mitigated the biochemical alterations and rescued axonal degeneration in patient iPSC-derived neurons. To further examine underlying disease mechanisms, we developed CYP7B1 knockout human embryonic stem cell (hESC) lines using CRISPR-cas9-mediated gene editing and, following differentiation, examined hESC-derived cortical PNs. Knockout of CYP7B1 resulted in similar axonal vesiculation and degeneration in human cortical PN axons, confirming a cause-effect relationship between gene deficiency and axonal degeneration. Interestingly, CYP7B1 deficiency led to impaired neurofilament expression and organization as well as axonal degeneration, which could be rescued with CDCA, establishing a new disease mechanism and therapeutic target to mitigate axonal degeneration.
Conclusions: Our data demonstrate disease-specific lipid disturbances and axonopathy mechanisms in human pluripotent stem cell-based neuronal models of CTX and SPG5 and identify CDCA, an established treatment of CTX, as a potential pharmacotherapy for SPG5. We propose this novel treatment strategy to rescue axonal degeneration in SPG5, a currently incurable condition.
Keywords: Axonal degeneration; Cerebrotendinous xanthomatosis; Chenodeoxycholic acid; Induced pluripotent stem cell; Spastic paraplegia type 5.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures

Similar articles
-
Impaired lipid metabolism in astrocytes underlies degeneration of cortical projection neurons in hereditary spastic paraplegia.Acta Neuropathol Commun. 2020 Dec 7;8(1):214. doi: 10.1186/s40478-020-01088-0. Acta Neuropathol Commun. 2020. PMID: 33287888 Free PMC article.
-
Inhibiting mitochondrial fission rescues degeneration in hereditary spastic paraplegia neurons.Brain. 2022 Nov 21;145(11):4016-4031. doi: 10.1093/brain/awab488. Brain. 2022. PMID: 35026838 Free PMC article.
-
Hereditary spastic paraplegia type 5: natural history, biomarkers and a randomized controlled trial.Brain. 2017 Dec 1;140(12):3112-3127. doi: 10.1093/brain/awx273. Brain. 2017. PMID: 29126212 Free PMC article. Clinical Trial.
-
Chenodeoxycholic Acid: An Update on Its Therapeutic Applications.Handb Exp Pharmacol. 2019;256:265-282. doi: 10.1007/164_2019_226. Handb Exp Pharmacol. 2019. PMID: 31267167 Review.
-
Epidemiology, diagnosis, and treatment of cerebrotendinous xanthomatosis (CTX).J Inherit Metab Dis. 2017 Nov;40(6):771-781. doi: 10.1007/s10545-017-0093-8. Epub 2017 Oct 4. J Inherit Metab Dis. 2017. PMID: 28980151 Review.
Cited by
-
Cerebrotendinous xanthomatosis with tremor as the main manifestation: A case report.Medicine (Baltimore). 2024 Apr 26;103(17):e37976. doi: 10.1097/MD.0000000000037976. Medicine (Baltimore). 2024. PMID: 38669366 Free PMC article.
-
Pluripotent Stem Cells as a Preclinical Cellular Model for Studying Hereditary Spastic Paraplegias.Int J Mol Sci. 2024 Feb 23;25(5):2615. doi: 10.3390/ijms25052615. Int J Mol Sci. 2024. PMID: 38473862 Free PMC article. Review.
-
A novel method for clustering cellular data to improve classification.Neural Regen Res. 2025 Sep 1;20(9):2697-2705. doi: 10.4103/NRR.NRR-D-24-00532. Epub 2024 Sep 24. Neural Regen Res. 2025. PMID: 39314166 Free PMC article.
-
Clinical and genetic analysis of a family with cerebrotendinous xanthomatosis.Front Neurol. 2025 May 27;16:1566740. doi: 10.3389/fneur.2025.1566740. eCollection 2025. Front Neurol. 2025. PMID: 40496134 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

